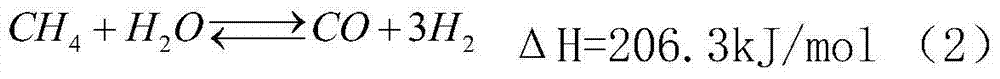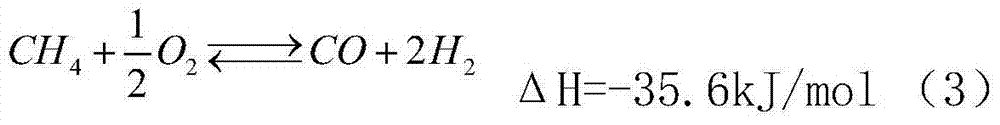Method and device for methane reforming with chemical chain CO2 by applying CO2 to dimethyl ether synthesis
A dimethyl ether and chemical chain technology, applied in the field of dimethyl ether synthesis, can solve the problems of low conversion rate of reactants, deactivation of catalyst carbon deposition, high reaction energy consumption, etc., to achieve improved recycling rate, efficient conversion and utilization, and improved The effect of reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
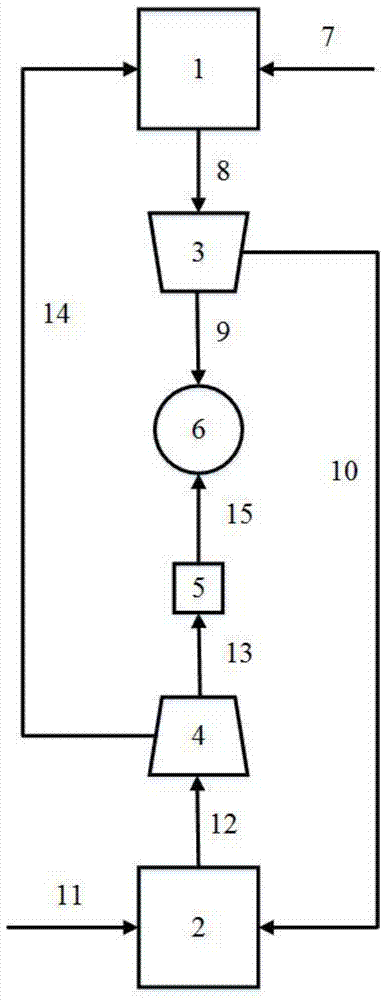
Image
Examples
Embodiment 1
[0041] CH 4 The feed flow rate is set to 1kmol / h, and the feed temperature is set to 850°C; CH 4 The temperature of the oxidation reactor is set to 850°C and the pressure is set to 1 atm; the oxygen carrier Fe 3 o 4 The circulating flow rate is set to 250kg / h; CO 2 The feed flow rate is set to 3.3kmol / h, and the feed temperature is set to 170°C; CO 2 The temperature of the reduction reactor was set to 170° C., and the pressure was set to 1 atm. The result of the calculation is: CH 4 The conversion rate is 97.91%, CO 2 The conversion ratio is 32.76%, and the hydrogen-to-carbon ratio (H 2 / CO) was 0.93.
Embodiment 2
[0043] CH 4 The feed flow rate is set to 1kmol / h, and the feed temperature is set to 800°C; CH 4 The temperature of the oxidation reactor is set to 800°C and the pressure is set to 5atm; the oxygen carrier Fe 3 o 4 The circulating flow rate is set to 300kg / h; CO 2 The feed flow rate is set to 3.3kmol / h, and the feed temperature is set to 100°C; CO 2 The temperature of the reduction reactor was set to 100° C., and the pressure was set to 5 atm. The result of the calculation is: CH 4 The conversion rate is 85.46%, CO 2 The conversion ratio is 39.26%, and the hydrogen-carbon ratio (H 2 / CO) was 0.71.
Embodiment 3
[0045] CH 4 The feed flow rate is set to 1kmol / h, and the feed temperature is set to 900°C; CH 4 The temperature of the oxidation reactor is set to 900°C and the pressure is set to 1 atm; the oxygen carrier Fe 3 o 4 The circulating flow rate is set to 200kg / h; CO 2 The feed flow rate is set to 3kmol / h, and the feed temperature is set to 200°C; CO 2 The temperature of the reduction reactor was set to 200° C., and the pressure was set to 1 atm. The result of the calculation is: CH 4 The conversion rate was 79.09%, CO 2 The conversion rate is 26.44%, and the hydrogen-carbon ratio (H 2 / CO) was 0.998.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com