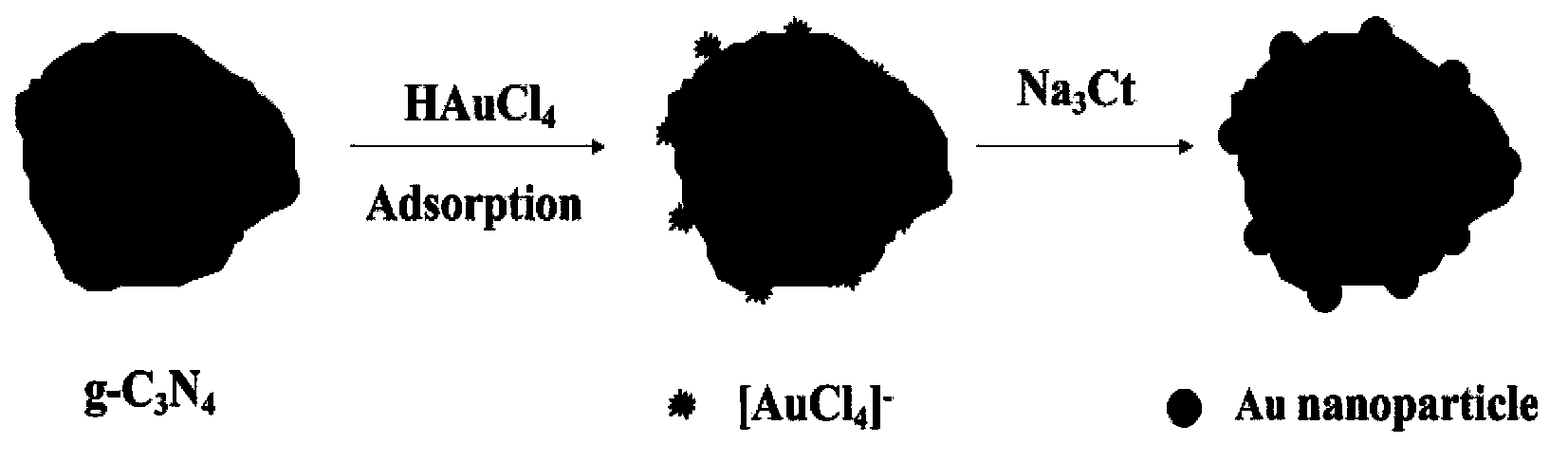
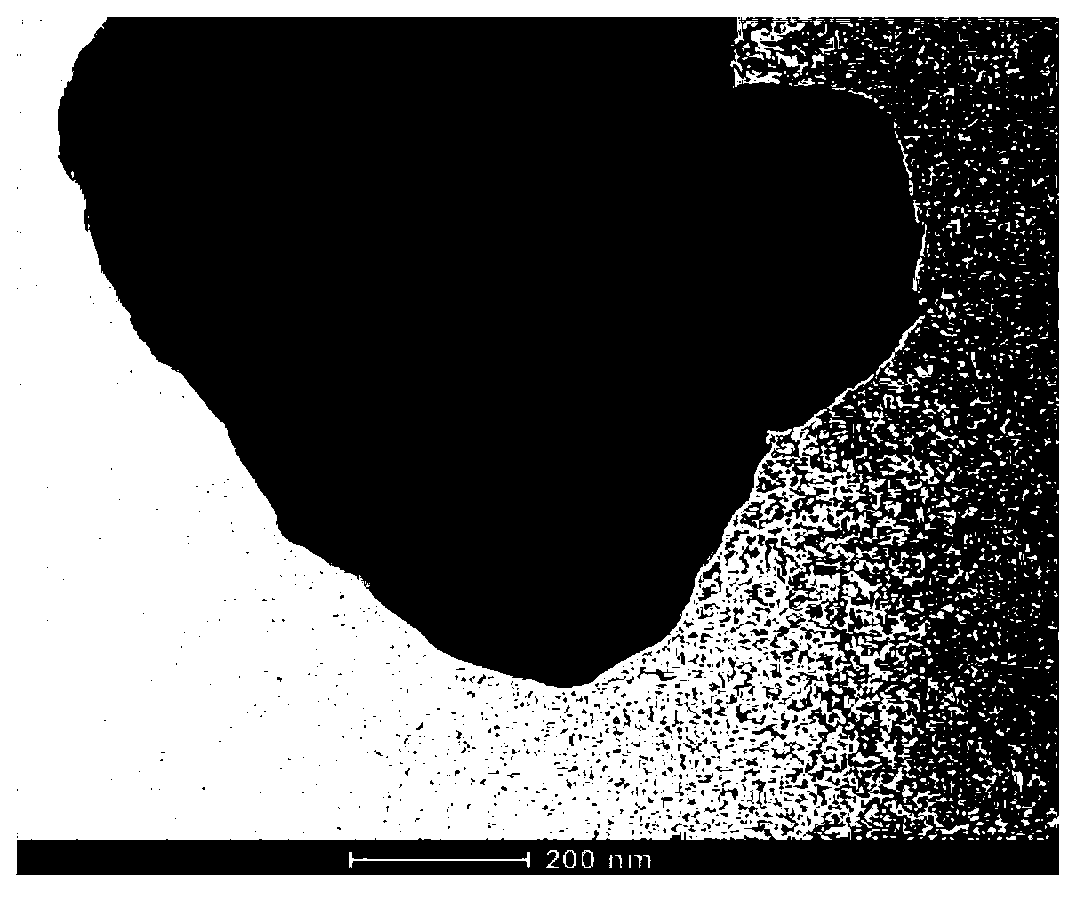
Method for preparing Au/g-C3N4 composite-type micro-nano material
A g-c3n4, micro-nano technology, applied in the field of material science, can solve problems such as complex preparation process, achieve the effects of simple production process, lower reaction energy barrier, and inhibit CO poisoning phenomenon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Weigh 2g of cyanamide into an alumina crucible, place the crucible in a muffle furnace, and -1 The temperature was raised to 400°C at the heating rate. When the set temperature is reached, the constant temperature reaction is carried out for 7h. Then naturally cooled to room temperature, the final yellow powder object is g-C 3 N 4 . Nitrogen protection was used during the reaction (nitrogen flow rate was 10 mL min -1 ). Yellow powder g-C 3 N 4 Grind in an agate mortar for 0.5 h for later use.
[0027] Weigh 0.12g g-C 3 N 4 Mix in 35mL HAuCl 4 4H 2 O (concentration: 2mM), disperse it evenly by ultrasonication for 5min to obtain a yellow suspension, then magnetically stir for 60min at a stirring speed of 1000r min -1 . Add 265 mL of deionized water to dilute the above suspension, and then transfer it to a water bath at 50°C, the color of the suspension turns light yellow. Under vigorous stirring (stirring speed is 1500r min -1 ) was added dropwise with 3.9m...
Embodiment 2
[0029] Weigh 2g of cyanamide into an alumina crucible, place the crucible in a muffle furnace, -1 The temperature was raised to 600°C at the heating rate. When the set temperature is reached, the constant temperature reaction is carried out for 5h. Then naturally cooled to room temperature, the final yellow powder object is g-C 3 N 4 . Use nitrogen protection during the reaction (nitrogen flow rate is 15mL min -1 ). Yellow powder g-C 3 N4 Grind in an agate mortar for 1 h for later use.
[0030] Weigh 0.1g g-C 3 N 4 Mix in 25mL HAuCl 4 4H 2 O (concentration: 3mM), disperse it evenly by ultrasonication for 8min to obtain a yellow suspension, then magnetically stir for 40min at a stirring speed of 1500r min -1 . Add 275 mL of deionized water to dilute the above suspension, and then transfer it to a water bath at 65°C, the color of the suspension turns light yellow. Under vigorous stirring (stirring speed is 2000r min -1 ) was added dropwise with 3.5mL Na 3 Ct (conc...
Embodiment 3
[0040] Weigh 2g of cyanamide into an alumina crucible, place the crucible in a muffle furnace, -1 The temperature was raised to 700°C at the heating rate. When the set temperature is reached, the constant temperature reaction is carried out for 3h. Then naturally cooled to room temperature, the final yellow powder object is g-C 3 N 4 . Use nitrogen protection during the reaction (nitrogen flow rate is 20mL min -1 ). Yellow powder g-C 3 N 4 Grind in an agate mortar for 2h for later use.
[0041] Weigh 0.09g g-C 3 N 4 Mix in 18mL HAuCl 4 4H 2 O (concentration: 4mM), disperse it evenly by ultrasonication for 10min to obtain a yellow suspension, then magnetically stir for 30min at a stirring speed of 1500r min -1 . Add 282 mL of deionized water to dilute the above suspension, and then transfer it to a water bath at 80°C, the color of the suspension turns light yellow. Under vigorous stirring (stirring speed is 3000r min -1 ) was added dropwise with 2.9mLNa 3 Ct (co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com