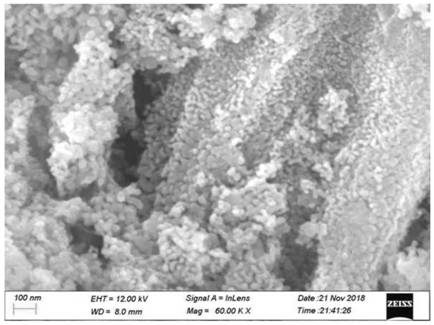
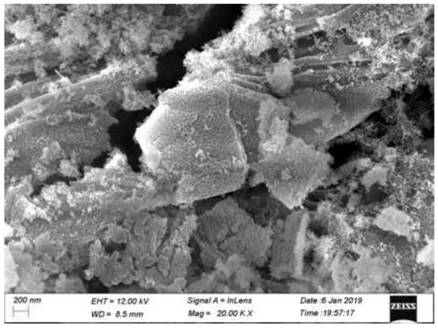
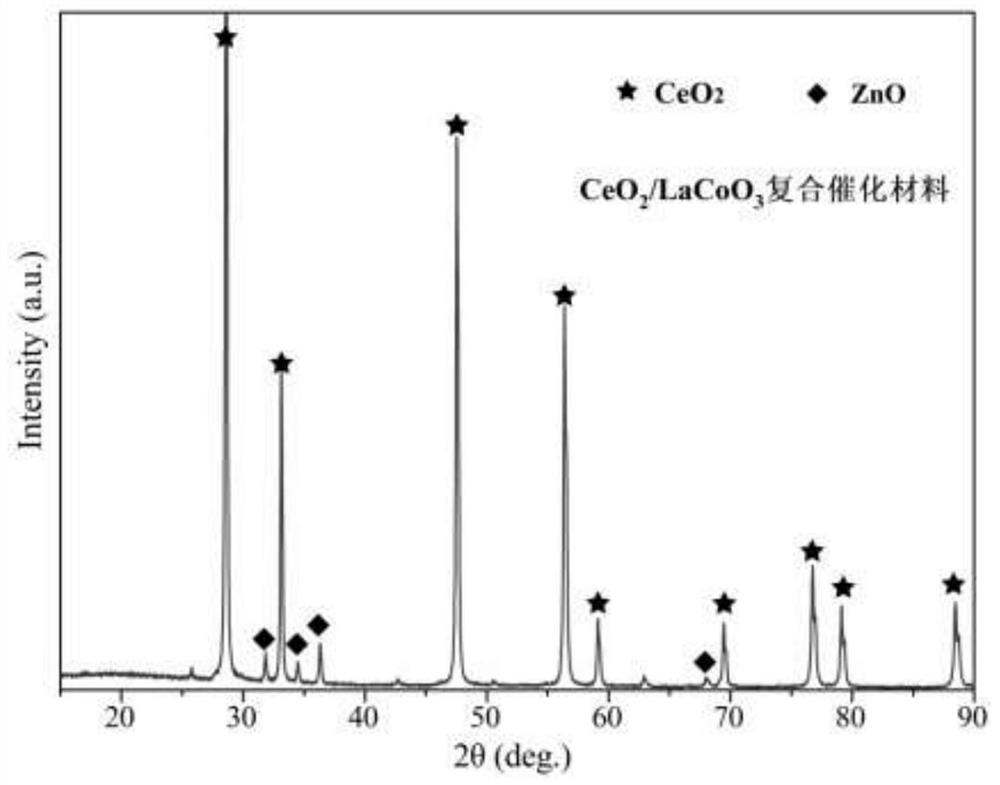
Chemical corrosion preparation method of porous CeO2 loaded perovskite composite catalytic material
A catalytic material and chemical corrosion technology, applied in the direction of catalyst activation/preparation, chemical instruments and methods, heterogeneous catalyst chemical elements, etc., can solve problems such as weak binding force, catalyst cannot be heat-treated at high temperature, complex and time-consuming process, etc., to achieve The effect of guaranteeing the service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The catalytic material prepared in this embodiment is CeO 2 Loaded LaCoO 3 , and using ZnO as the preparation process of the sacrificial oxide, the specific preparation process is as follows:
[0045] (1) Configuration solution:
[0046] Configure Ce(NO) with a molar ratio of 2:0.1:0.1:2:4.8:2 respectively 3 、La(NO) 3 、Co(NO) 3 , Zn(NO) 2 、K 2 (CO) 3 and NaOH aqueous solution for subsequent use;
[0047] (2) Preparation of precursor:
[0048] The above Ce(NO) 3 、La(NO) 3 、Co(NO) 3 and Zn(NO) 2 Mix the solution evenly, and stir it magnetically until it is evenly mixed, then slowly add the configured K 2 (CO) 3 solution, when the initial precipitation occurs, add the configured NaOH aqueous solution, and after the precipitation is complete, filter the precipitate, wash it to neutrality, and dry it at a temperature of 60°C, and then keep it warm at 400°C for 3 hours, CeO was formed after incubation at 600°C for 9 hours 2 / LaCoO 3 / ZnO precursor;
[0049] ...
Embodiment 2
[0054] This embodiment is basically the same as Embodiment 1, the difference is:
[0055] The catalytic material prepared in this embodiment is CeO 2 Loaded LaCoO 3 , and with Al 2 o 3 As a preparation process for sacrificial oxides, the specific preparation process is as follows:
[0056] (1) Configuration solution:
[0057] Configure Ce(NO) with a molar ratio of 2:0.1:0.1:2:4.8:2 respectively 3 、La(NO) 3 、Co(NO) 3 , Al(NO) 3 、K 2 (CO) 3 and NaOH aqueous solution for subsequent use;
[0058] (2) Preparation of precursor:
[0059] The above Ce(NO) 3 、La(NO) 3 、Co(NO) 3 and Al(NO) 3 Mix the solution evenly, and stir it magnetically until it is evenly mixed, then slowly add the configured K 2 (CO) 3 solution, when the initial precipitation occurs, add the configured NaOH aqueous solution, and after the precipitation is complete, filter the precipitate, wash it to neutrality, and dry it at a temperature of 60°C, and then keep it warm at 400°C for 3 hours, CeO wa...
Embodiment 3
[0065] This embodiment is basically the same as Embodiment 1, the difference is:
[0066] The catalytic material prepared in this embodiment is CeO 2 Loaded LaMnO 3 , and with Al 2 o 3 As a preparation process for sacrificial oxides, the specific preparation process is as follows:
[0067] (1) Configuration solution:
[0068] Configure Ce(NO) with a molar ratio of 2:0.1:0.1:2:4.8:2 respectively 3 、La(NO) 3 , Mn(NO) 2 , Al(NO) 3 、K 2 (CO) 3 and NaOH aqueous solution for subsequent use;
[0069] (2) Preparation of precursor:
[0070] The above Ce(NO) 3 、La(NO) 3 , Mn(NO) 2 and Al(NO) 3 Mix the solution evenly, and stir it magnetically until it is evenly mixed, then slowly add the configured K 2 (CO) 3 solution, when the initial precipitation occurs, add the configured NaOH aqueous solution, and after the precipitation is complete, filter the precipitate, wash it to neutrality, and dry it at a temperature of 60°C, and then keep it warm at 400°C for 3 hours, CeO ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com