Amphiphilic porphyrin-based photosensitizer and preparation and applications thereof
A porphyrin-based photosensitizer and amphiphilic technology, applied in the field of biomedicine, can solve the problems of poor biocompatibility and tumor targeting, and achieve the effect of good tumor cell uptake rate and efficient uptake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
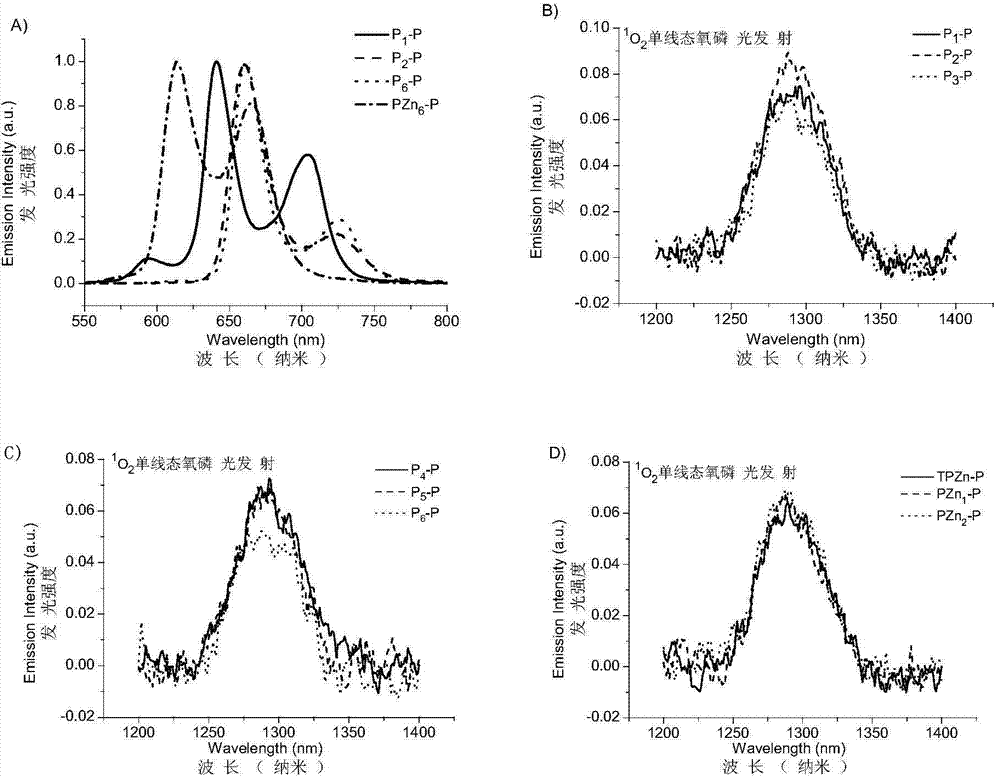
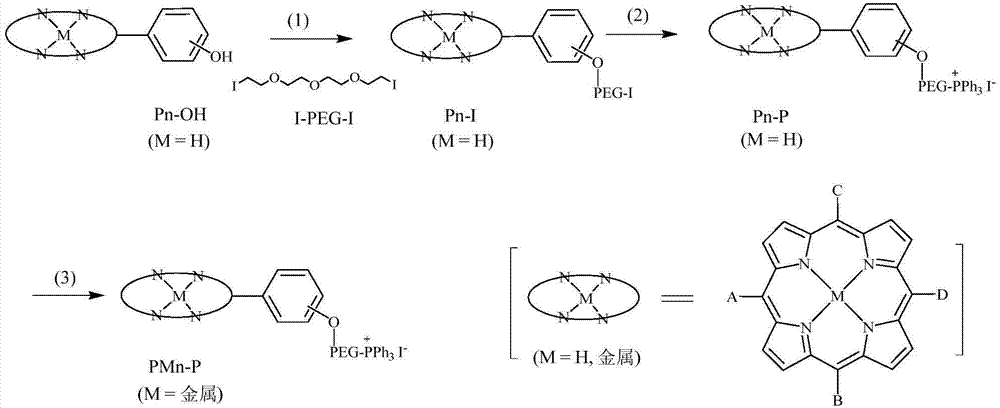
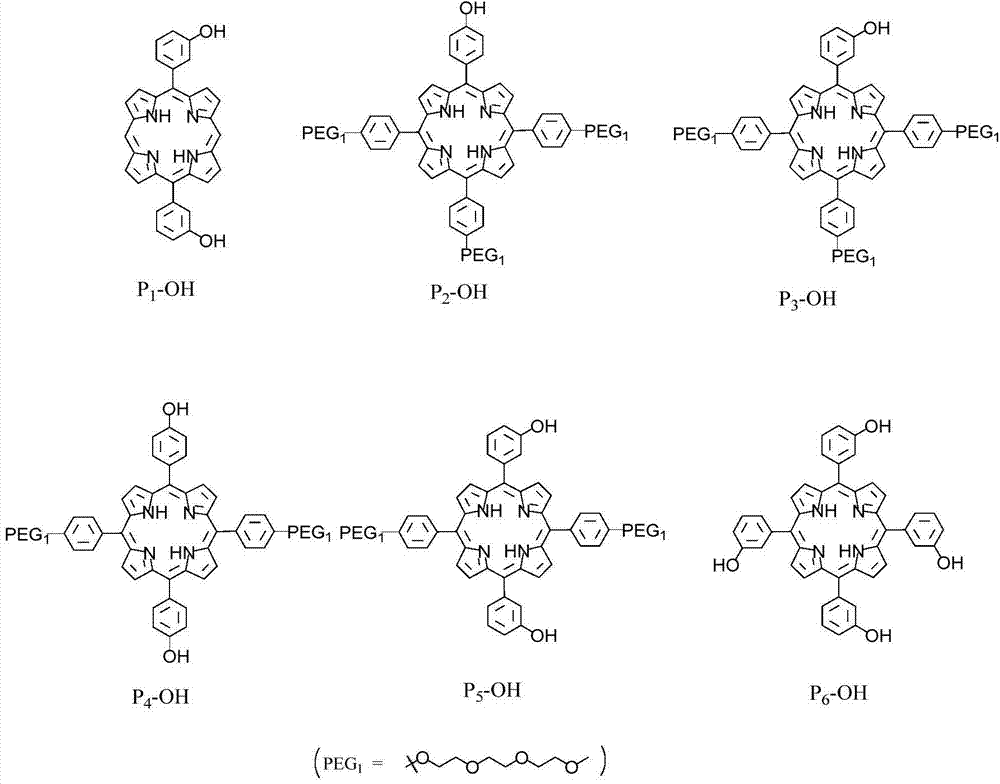
[0037] (1) Under the protection of nitrogen, the porphyrin raw material 49.5mg (0.1mmol) P 1 -OH(5,15-bis(4-hydroxyphenyl)-21H,23H-porphyrin)( figure 2 shown in), 207.0mg (0.5mmol) diiodotriethylene glycol (I-PEG-I) ( figure 1 shown in ) and 138mg (1.0mmol) potassium carbonate (K 2 CO 3 ) was dissolved in 5mL of anhydrous DMF, the temperature was raised to 65°C, the reaction was terminated by TLC detection, and the solvent was removed to obtain a crude product, which was separated with a silica gel chromatographic column to obtain the purple porphyrin intermediate P 1 -I, structural formula such as image 3 As shown in , the yield was 88%.
[0038] Characterization data: 1 HNMR (CDCl 3 ):δ-3.15(s,2H),3.12(t,J=6.76Hz,4H),3.52(m,4H),3.58(m,8H),3.67(m,4H),3.75(dt,J= 5.24Hz, J=6.36Hz, 4H), 3.94(t, J=4.6Hz, 4H), 4.34(t, J=4.66Hz, 4H), 7.36(dd, J=1.64Hz, J=6.08Hz, 2H ),7.66(t,J=7.82Hz,2H),7.85(d,J=2.4Hz,3H),7.87(s,1H),9.10(d,J=4.56Hz,4H),9.37(d,J =4.60Hz, 4H), 10.30(s, 2H)...
Embodiment 2
[0044] (1) Under nitrogen protection, 56.0mg (0.05mmol) Porphyrin raw material 2 -OH(5,10,15-tris(4-(triethylene glycol monomethyl ether))-20-(4-hydroxyphenyl)-21H,23H-porphyrin)( figure 2 shown in), 103.5mg (0.25mmol) diiodotriethylene glycol (I-PEG-I) ( figure 1 shown in ) and 69mg (0.5mmol) of potassium carbonate (K 2 CO 3 ) was dissolved in 5mL of anhydrous DMF, the temperature was raised to 65°C, the reaction was terminated by TLC detection, and the solvent was removed to obtain a crude product, which was separated with a silica gel chromatographic column to obtain the purple porphyrin intermediate P 2 -I, structural formula such as image 3 As shown in , the yield was 81%.
[0045] Characterization data: 1 HNMR (CDCl 3 ):δ-2.76(s,2H),3.27(t,J=6.72Hz,2H),3.41(s,9H),3.60(m,8H),3.71(m,10H),3.81(m,10H) ,3.88(m,8H),4.09(t,J=4.98Hz,8H),4.42(m,8H),7.29(d,J=6.10Hz,8H),8.15(d,J=6.1Hz,8H) ,8.89(s,8H); MALDI-TOF MS:calcd.for[M + ]:1403.3936,found:1403.4206.
[0046] (2) ...
Embodiment 3
[0051] (1) Under nitrogen protection, 56.0mg (0.05mmol) Porphyrin raw material 3 -OH(5,10,15-tris(4-(triethylene glycol monomethyl ether))-20-(3-hydroxyphenyl)-21H,23H-porphyrin)( figure 2 shown in), 103.5mg (0.25mmol) diiodotriethylene glycol (I-PEG-I) ( figure 1 shown in ) and 69mg (0.5mmol) of potassium carbonate (K 2 CO 3 ) was dissolved in 5mL of anhydrous DMF, the temperature was raised to 65°C, the reaction was terminated by TLC detection, and the solvent was removed to obtain a crude product, which was separated with a silica gel chromatographic column to obtain the purple porphyrin intermediate P 3 -I, structural formula such as image 3 As shown in , the yield was 79%.
[0052] Characterization data: 1 HNMR (CDCl 3 ):δ-2.79(s,2H),3.13(t,J=6.76Hz,2H),3.43(s,9H),3.53(m,2H),3.60(m,10H),3.66(m,2H) ,3.73(m,8H),3.78(m,6H),3.87(m,6H),3.94(t,J=4.64Hz,2H),4.04(t,J=4.72Hz,6H),4.31(t, J=4.96Hz, 2H), 4.41(t, J=4.52Hz, 6H), 7.28(d, J=8.36Hz, 6H), 7.32(m, 1H), 7.61(t, J=7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com