Goose paramyxovirus chicken embryo fibroblast cell adapted strain and application thereof
A goose paramyxovirus and fibroblast technology, applied in antiviral agents, viruses/bacteriophages, medical preparations containing active ingredients, etc., can solve the problems of poor product quality between batches and high cost, and achieve stable product quality, Good immunogenicity and high antibody titer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
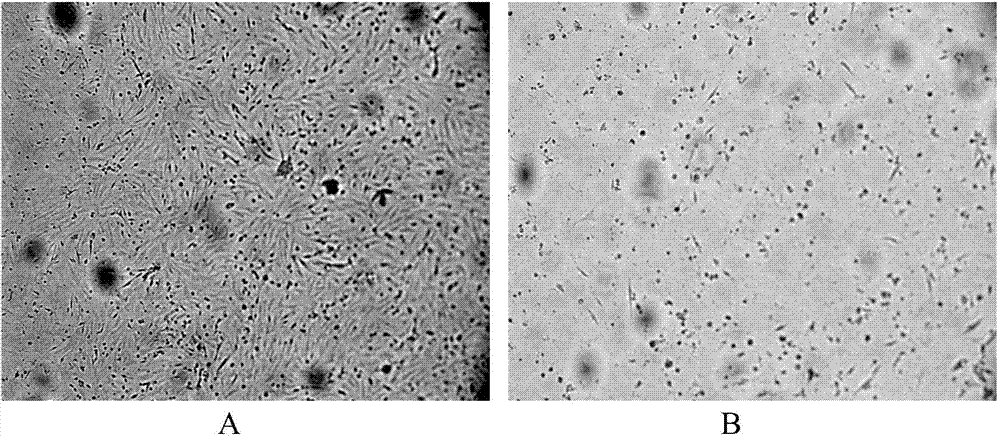
[0029] Example 1 Isolation and Identification of Goose Paramyxovirus
[0030] 1. Experimental method
[0031] 1.1 Isolation of virus
[0032] The disease material came from a farm in the suburbs of Daqing City, Heilongjiang Province. The liver, spleen and other disease materials of the dead goose were aseptically collected, mixed, chopped and ground, and sterilized PBS with a double antibody content of 2000IU / mL was added at a ratio of 1:3 (v / v) , placed in a refrigerator at 4°C for 4 hours, and centrifuged at 4000r / min for 5 minutes; take the supernatant and inoculate five 10-day-old SPF chicken embryos through the allantoic cavity, 0.2mL / piece, and incubate at 37°C for 120 hours; discard the dead chicken embryos within 24 hours, After that, the eggs were illuminated twice a day, and the dead chicken embryos were stored in a refrigerator at 4°C for 4-24 hours; the allantoic fluid and amniotic fluid of the dead chicken embryos were collected from 48h to 120h, and used as the ...
Embodiment 2
[0079] Embodiment 2 animal immune protection test
[0080] 1. Experimental method
[0081] 1.1 Preparation of oil emulsion inactivated vaccine
[0082] 1.1.1 Preparation of virus solution
[0083] The goose paramyxovirus DQ01 strain, DQ02 strain, DQ03 strain, DQ04 strain and DQ05 strain isolated in Example 1 were cultured by chicken embryo fibroblast spinner bottles respectively, and the cell culture virus liquid was collected to measure HA and TCID 50 In addition, the original strain that embodiment 1 isolates is inoculated into SPF chicken embryo (collects allantoic fluid), measures its HA and EID 50 .
[0084] 1.1.2 Inactivation and inactivation test of virus liquid
[0085] 1.1.2.1 Inactivation of virus fluid
[0086] Add the tested virus liquid to a final concentration of 0.1% formaldehyde solution for inactivation, and inactivate at 37°C for 24 hours.
[0087] 1.1.2.2 Inactivation test
[0088] (1) Inactivation test of cell virus liquid
[0089] Take the inactiva...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com