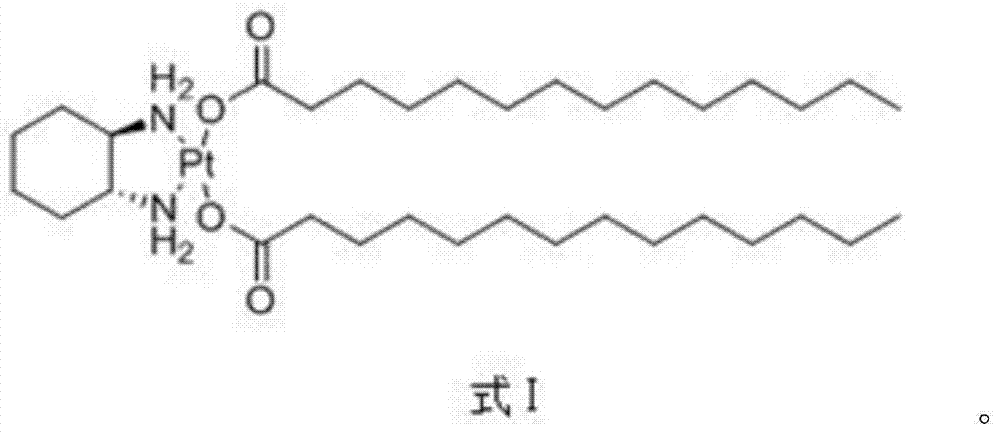
Miriplatin lyophilized preparation and preparation method thereof
A technology of freeze-dried preparations and rice platinum, which is applied in freeze-dried transportation, active ingredients of heavy metal compounds, powder transportation, etc., can solve the problems of unfavorable large-scale production, cumbersome production process, and increased production costs. It is difficult to achieve viscosity, enhance curative effect, Reduce the effect of embolism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
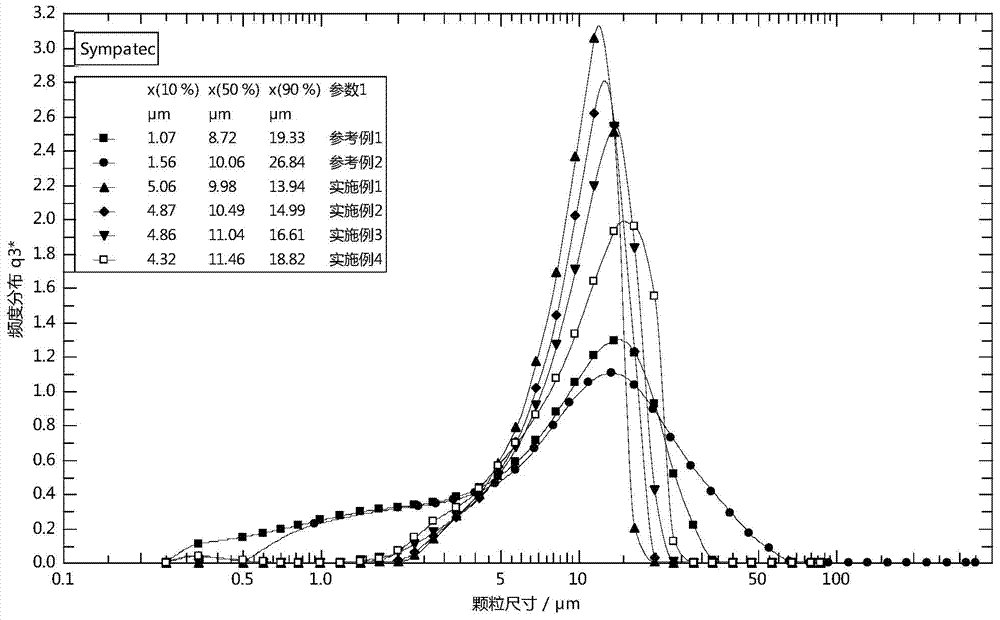
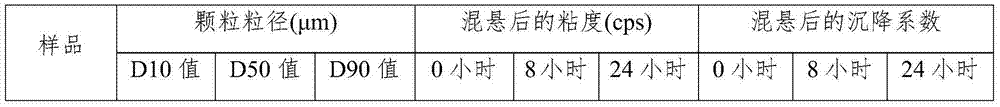
Image
Examples
reference example 1
[0042] Prescription (per 1000 bottles):
[0043] Mi Platinum
[0044] It can be prepared with reference to Chinese patent ZL02818527.7: take the prescribed amount of tert-butanol, add the prescribed amount of rice platinum, heat to 30-40 degrees, mix until the drug is completely dissolved, and adjust the water content of the solution under the monitoring of a Karl-Hugh moisture analyzer. To 1.5mg / mL, use a 0.22μm filter membrane to perform sterile filtration to obtain the intermediate of miplatin preparation.
reference example 2
[0046] Prescription (per 1000 bottles):
[0047] Mi Platinum
[0048] It can be prepared by referring to Chinese patent ZL201110201119.1: take the prescribed amount of tert-butanol, add the prescribed amount of rice platinum, heat to 30-40 degrees, mix until the drug is completely dissolved, add the prescribed amount of absolute ethanol, mix well, and use a 0.22 μm filter membrane Sterile filtration is carried out to obtain the intermediate of Miplatin preparation.
Embodiment 1
[0050] Prescription (per 1000 bottles):
[0051] Mi Platinum
[0052] Take the prescribed amount of tert-butanol, add the prescribed amount of miplatin, heat to 30-40°C, mix until the drug is completely dissolved, add the prescribed amount of water for injection and absolute ethanol, mix evenly, and use a 0.22 μm filter membrane to perform sterilizing filtration to obtain Miplatin preparation intermediate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com