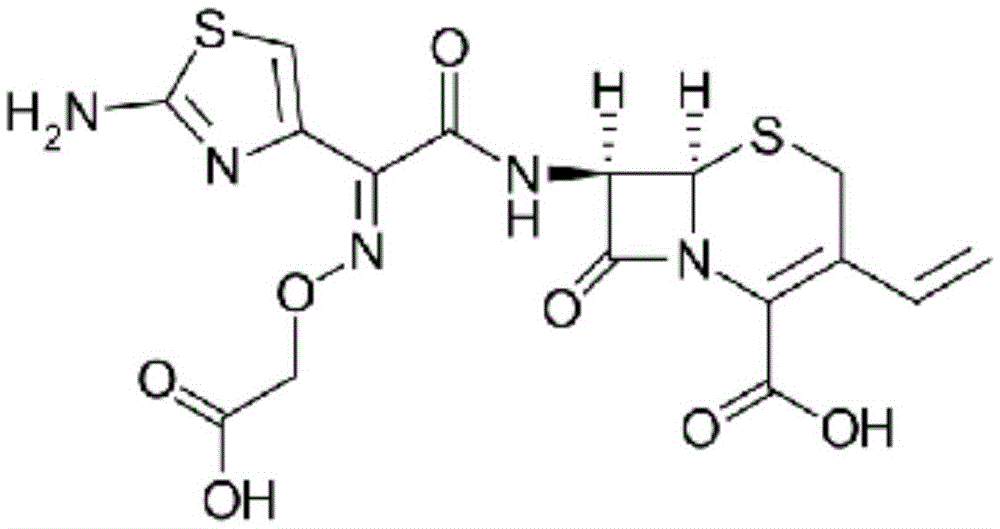
Cefixime granules and preparation method thereof
A technology of cefixime and granules, which is applied in the field of cefixime granules and its preparation, can solve the problems of too many granules, fine powder, inability to dissolve and disperse quickly, and unfavorable drug administration for children.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The preparation of embodiment 1 cefixime granules
[0050]
[0051] Process:
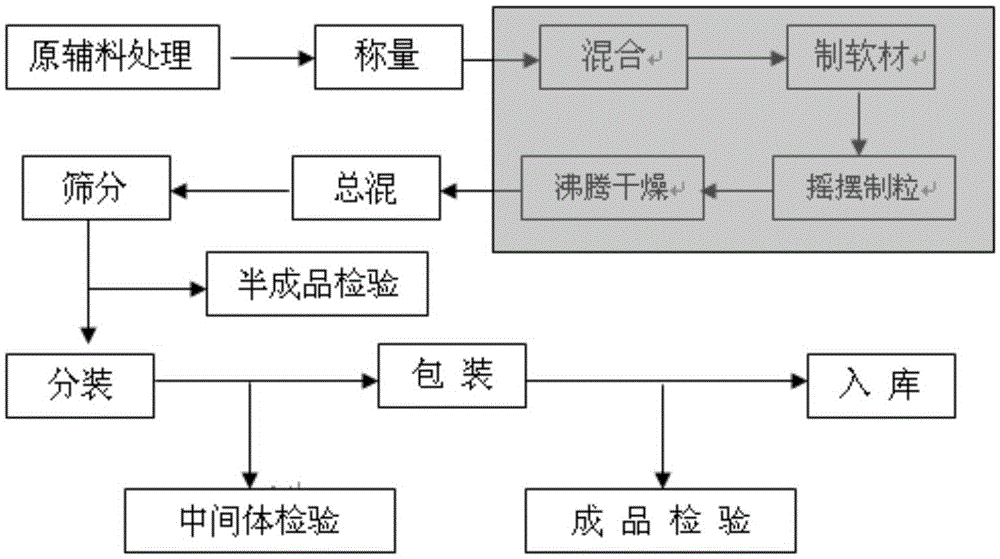
[0052] Take cefixime and pass through a 120-mesh sieve, and sucrose, hydroxypropyl cellulose, sodium citrate, citric acid, polyvinylpyrrolidone, neotame, orange essence, and chocolate essence respectively pass through a 60-mesh sieve, and set aside. Dissolve polyvinylpyrrolidone in purified water to prepare a 15% aqueous solution, and set aside.
[0053] Weigh the sieved cefixime, sucrose, hydroxypropyl cellulose, sodium citrate, citric acid and neotame into the fluidized bed for dry mixing and preheat until the material temperature reaches 50°C. The binder solution is sprayed in for granulation. Drying is carried out after the granulation is completed, and the temperature of the material is controlled not to be higher than 60°C for boiling drying. Sieve the dry granules with 10-mesh and 80-mesh sieves, put them into the mixer together with the prescribed amount of sieved orange essence...
Embodiment 2
[0054] The preparation of embodiment 2 cefixime granules
[0055]
[0056] Process:
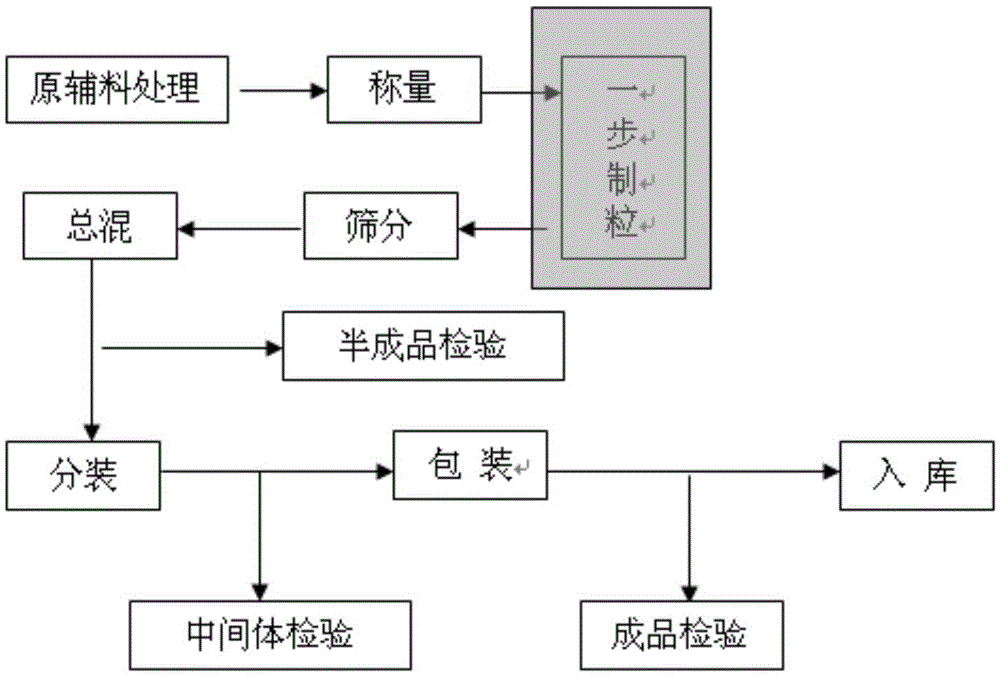
[0057] Pass cefixime through a 120-mesh sieve, and pass through a 60-mesh sieve for maltodextrin, starch, sodium citrate, citric acid, aspartame, pineapple essence, and sunset yellow, respectively, and set aside. Dissolve polyvinylpyrrolidone in purified water to prepare a 15% aqueous solution, and set aside.
[0058] Weigh the sieved cefixime, maltodextrin, starch, sodium citrate, citric acid, aspartame, and sunset yellow into the fluidized bed for dry mixing and preheat to the material temperature When the temperature reached 45°C, the binder solution was sprayed in for granulation. Drying is carried out after the granulation is completed, and the temperature of the material is controlled not to be higher than 60°C for boiling drying. Sieve the dry granules with 10-mesh and 80-mesh sieves, put them into the mixer together with the prescribed amount of sieved pineapple essence, mix the...
Embodiment 3
[0059] The preparation of embodiment 3 cefixime granules
[0060]
[0061] Process:
[0062] Pass cefixime through a 100-mesh sieve, lactose, sodium bicarbonate, sodium carbonate, stevioside, strawberry essence, and lemon yellow through a 80-mesh sieve respectively, and set aside. Sodium carboxymethyl cellulose was dissolved in purified water to prepare a 5% aqueous solution for subsequent use.
[0063] Weigh the sieved cefixime, lactose, sodium bicarbonate, sodium carbonate, stevioside and lemon yellow into the fluidized bed for dry mixing, preheat until the material temperature reaches 40°C and start spraying Dosage solution for granulation. Drying is carried out after the granulation is completed, and the temperature of the material is controlled not to be higher than 60°C for boiling drying. Sieve the dry granules with 10-mesh and 80-mesh sieves, put them into the mixer together with the sieved strawberry essence of the prescription, mix them evenly, take samples for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com