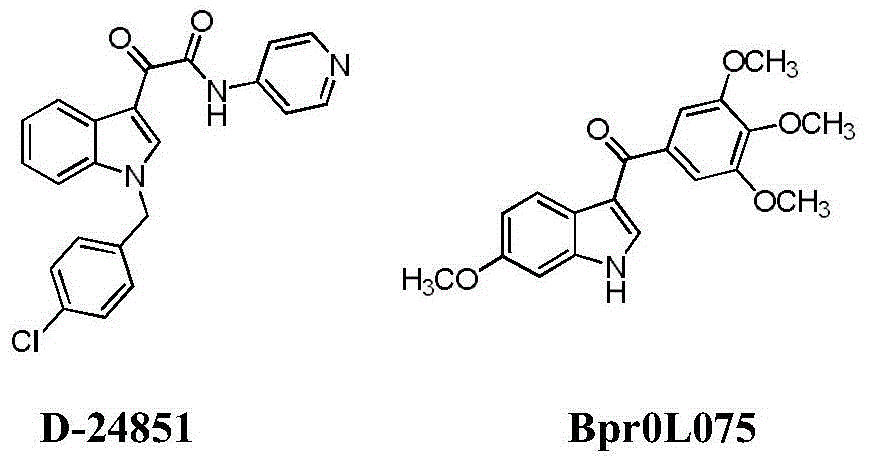
Indole compounds containing alpha-methylene-gamma-butyrolactone structures, preparation method and application thereof
A compound, butyrolactone technology, applied in the application of tubulin inhibitors in anti-tumor activity, the field of new indole compounds containing α-methylene-γ-butyrolactone structure, which can solve the difficulty of synthesis Large, normal cell effects, neurotoxicity, etc., to achieve short steps, high yield, good activity and broad-spectrum effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
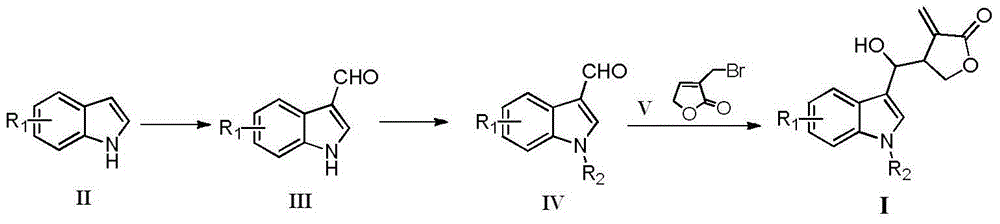
Image
Examples
Embodiment 1
[0025] Example 1 As shown in the general formula (III), R 1 Preparation of derivative (III-1) of =-H
[0026] Add 40mL DMF into the round bottom flask, slowly add POCl dropwise under ice bath 3 (11.9mL0.128mol), keep stirring in an ice bath for 30min after the dropwise addition, then add dropwise a DMF (20mL) solution of indole (10g, 0.085mol), keep the reaction at room temperature for 3h after dropping, then add 300mL of ice water, and then drop Add 10% by mass NaOH aqueous solution, adjust the pH to 7-8, stir at room temperature overnight, a large amount of white solid precipitates, filter with suction, wash, and dry to obtain 10.74 g of compound III-1, with a yield of 86.7%.
Embodiment 2
[0027] Example 2 As shown in general formula (III), R 1 = Preparation of derivative (III-2) of 5-Cl
[0028] Take the same method as in Example 1 to prepare III-2, R 1 = 5-Cl.
[0029] Add 40mL DMF into the round bottom flask, slowly add POCl dropwise under ice bath 3 (9.2mL0.099mol), keep stirring in an ice bath for 30min after the dropwise addition, then add dropwise a solution of 5-chloroindole (10g, 0.066mol) in DMF (20mL), keep the reaction at room temperature for 3h after dropping, then add 300mL of ice water , and then dropwise added 10% NaOH aqueous solution to adjust the pH to 7-8, stirred overnight at room temperature, a large amount of white solids were precipitated, and were filtered and dried to obtain 10.02 g of compound III-2, with a yield of 84.5%.
Embodiment 3
[0030] Example 3 As shown in general formula (IV), R 1=-H,R 2 = Preparation of derivative (IV-1) of p-F-Bn- After concentrated under reduced pressure, recrystallized with ethanol, and suction filtered to obtain 1.33 g of white solid (IV-1), yield: 76.3%. 1 H NMR (400MHz, CDCl 3 )δ:10.01(s,1H),8.40-8.29(m,1H),7.71(s,1H),7.48-7.26(m,3H),7.21-7.12(m,2H),7.12-6.96(m, 2H), 5.34(s, 2H); 13 C NMR (101MHz, CDCl 3 )δ184.62, 138.26, 131.14, 129.05, 128.96, 125.55, 124.27, 123.18, 122.25, 118.63, 116.26, 116.05, 110.30, 50.27.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com