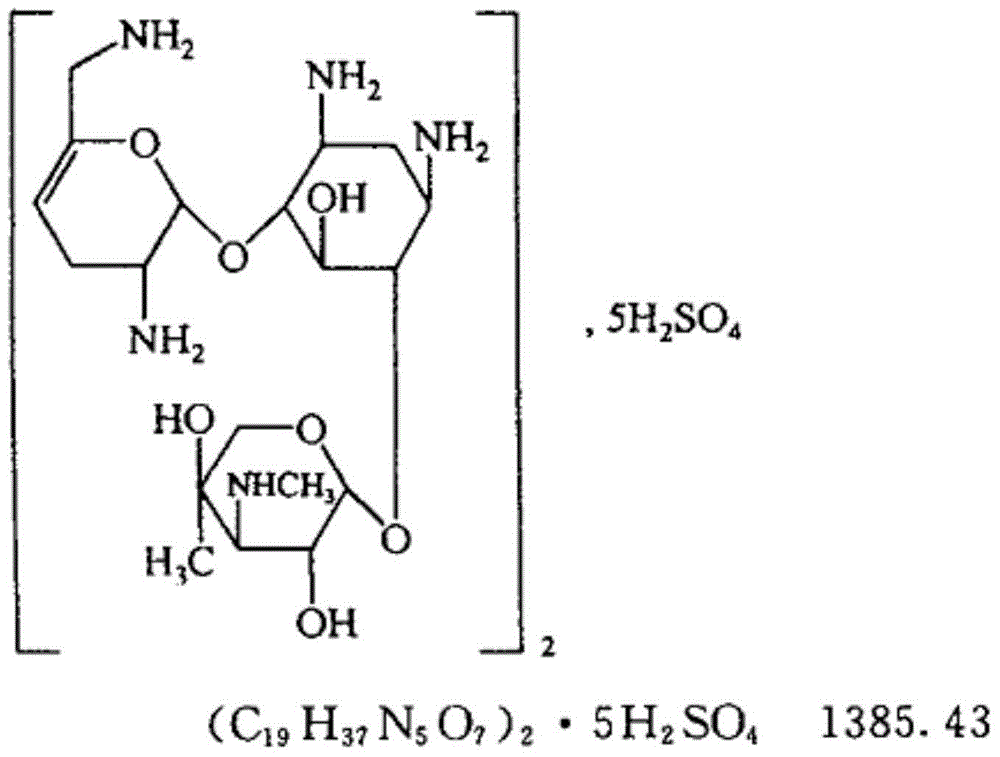
Pharmaceutical composition of sisomicin sulphate injection and preparation method
A technology of sisomicin sulfate and injection, applied in drug delivery, pharmaceutical formula, antibacterial drugs, etc., can solve the problems of fermentation process optimization and process control research lag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0214] Embodiment 1: Preparation of Sisomicin Sulfate Injection
[0215] formula:
[0216] Sisomicin Sulfate 500 00U,
[0218] L-cysteine 1mg,
[0219] Edetate Disodium 0.4mg,
[0220] Water for injection, add appropriate amount to 1ml.
[0221] Preparation method:
[0222] (1) Dissolve the pretreated sisomicin sulfate and edetate disodium of the prescription quantity in an appropriate amount of water for injection (70% of the formula quantity), add sodium bisulfite and L-cysteine of the prescription quantity acid, stir to dissolve;
[0223] (2) If necessary, use an acid-base regulator to adjust the pH value of the solution to between 5.2 and 5.5, add 0.2% activated carbon, stir and absorb for 30 minutes, decarbonize, and add water for injection to the full amount of the formula;
[0224] (3) Filter the prepared medicinal solution through a 0.45 μm microporous membrane, then sterilize and filter through a 0.22 μm microporous membrane, ...
Embodiment 2
[0225] Embodiment 2: Preparation of Sisomicin Sulfate Injection
[0226] formula:
[0227] Sisomicin Sulfate 600 00U,
[0229] L-cysteine 1.2mg,
[0230] Edetate Disodium 0.3mg,
[0231] Water for injection, add appropriate amount to 1ml.
[0232] Preparation method:
[0233] (1) Dissolve the pretreated sisomicin sulfate and edetate disodium of the prescription quantity in an appropriate amount of water for injection (80% of the formula quantity), add sodium bisulfite and L-cysteine of the prescription quantity acid, stir to dissolve;
[0234] (2) If necessary, use an acid-base regulator to adjust the pH value of the solution to between 5.2 and 5.5, add 0.2% activated carbon, stir and absorb for 30 minutes, decarbonize, and add water for injection to the full amount of the formula;
[0235] (3) Filter the prepared medicinal solution through a 0.45 μm microporous membrane, then sterilize and filter through a 0.22 μm microporous membran...
Embodiment 3
[0236] Embodiment 3: preparation sisomicin sulfate injection
[0237] formula:
[0238] Sisomicin Sulfate 400 00U,
[0239] Sodium bisulfite 6mg,
[0240] L-cysteine 0.8mg,
[0241] Edetate Disodium 0.5mg,
[0242] Water for injection, add appropriate amount to 1ml.
[0243] Preparation method:
[0244] (1) Dissolve the pretreated sisomicin sulfate and edetate disodium of the prescription quantity in an appropriate amount of water for injection (60% of the formula quantity), add sodium bisulfite and L-cysteine of the prescription quantity acid, stir to dissolve;
[0245] (2) If necessary, use an acid-base regulator to adjust the pH value of the solution to between 5.2 and 5.5, add 0.2% activated carbon, stir and absorb for 30 minutes, decarbonize, and add water for injection to the full amount of the formula;
[0246] (3) Filter the prepared medicinal solution through a 0.45 μm microporous membrane, then sterilize and filter through a 0.22 μm microporous membrane, d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com