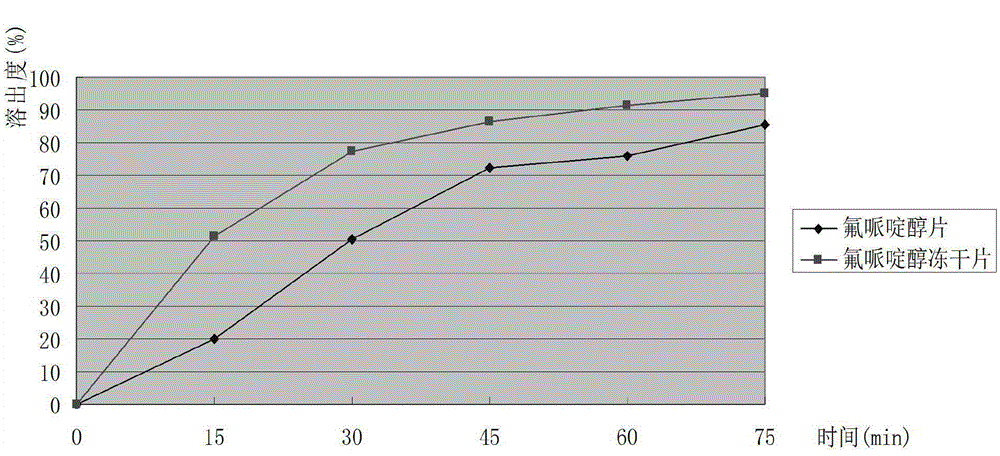
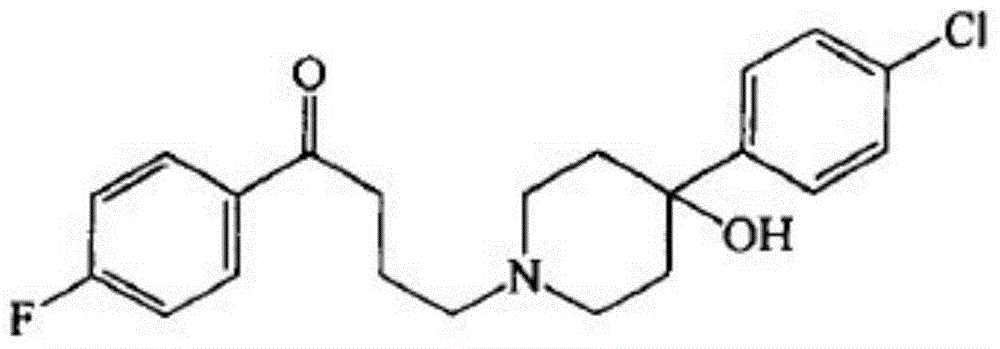
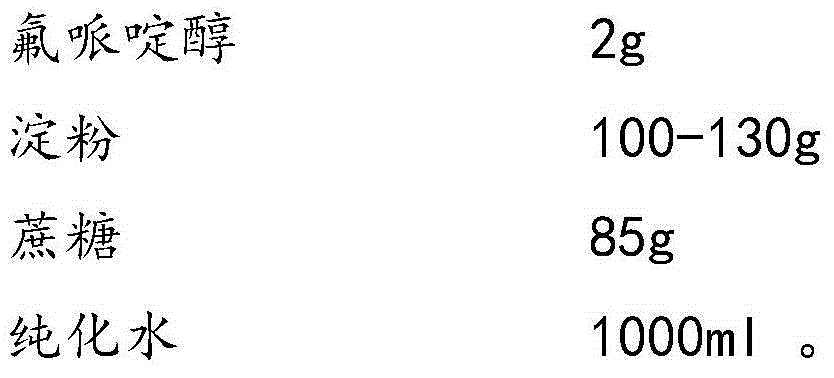Haloperidol composition freeze drying tablet and preparation method thereof
The technology of haloperidol and composition is applied in the field of haloperidol composition freeze-dried tablet and preparation thereof, and achieves the effects of ensuring curative effect and safety, large dissolution rate and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
[0027] A. Weigh 100g of cornstarch, add 900ml of purified water and stir, use a pH regulator to control the pH of the solution at 5-7.5, then heat to 72°C and keep for 120 minutes to make 9% (W / V) cornstarch solution.
[0028] B. Measure 45ml of purified water, boil, add 85g of sucrose, stir, after dissolving, continue heating to 100°C, filter with refined cotton, wash the filter with an appropriate amount of hot distilled water, combine the lotion and filtrate, let cool, add Add appropriate amount of distilled water to make the whole volume into 100mL, stir well to obtain B solution.
[0029] C. Mix the solution obtained in step A with the solution obtained in step B, fully stir for 30 minutes, and then lower the solution to normal temperature to obtain a corn-sucrose solution.
[0030] D. Weigh 2 g of haloperidol, add it into 1 L of corn-sucrose solution, and stir for 30 minutes.
[0031] E. After measuring the content of haloperidol in the medicinal solution, ...
Embodiment 2
[0034]
[0035] A. Weigh 130g of cornstarch, add 900ml of purified water and stir, use a pH regulator to control the pH of the solution at 5-7, then heat to 72°C and keep for 120 minutes to make 13% (W / V ) of cornstarch solution.
[0036] B. Measure 45ml of purified water, boil, add 85g of sucrose, stir, after dissolving, continue heating to 100°C, filter with refined cotton, wash the filter with an appropriate amount of hot distilled water, combine the lotion and filtrate, let cool, add Add appropriate amount of distilled water to make the whole volume into 100mL, stir well to obtain B solution.
[0037] C. Mix the solution obtained in step A with the solution obtained in step B, fully stir for 30 minutes, and then lower the solution to normal temperature to obtain a corn-sucrose solution.
[0038] D. Weigh 2 grams of haloperidol (calculated as 1000 tablets), add 1L of corn-sucrose solution, and stir for 30 minutes.
[0039] E. After measuring the content of haloperidol ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com