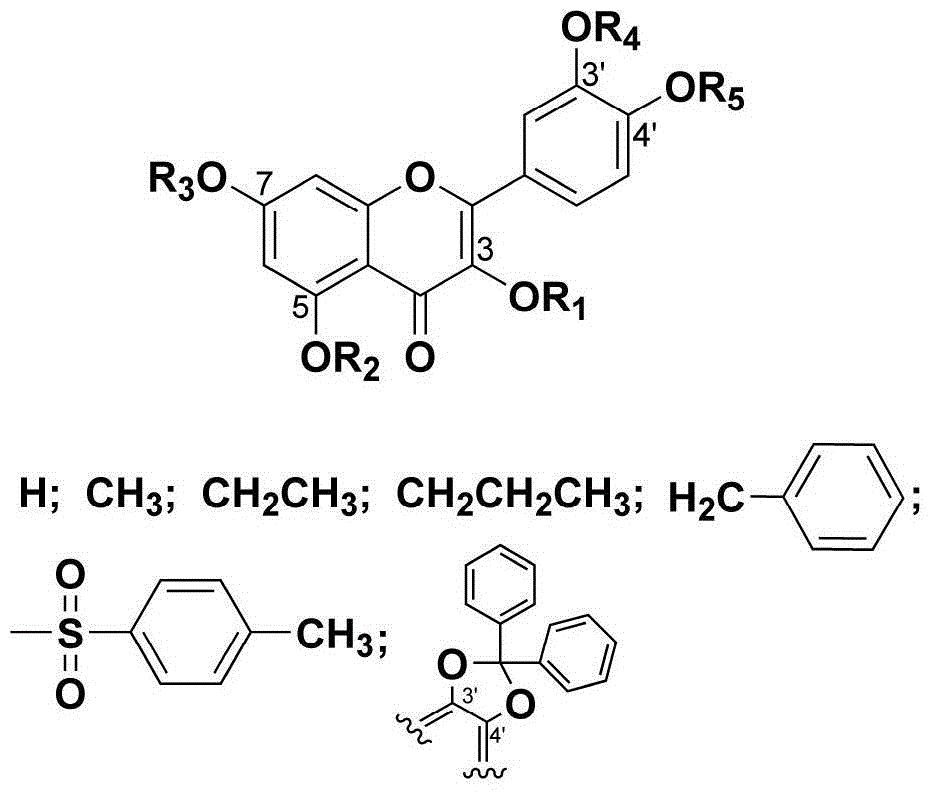
Quercetin derivative and preparation method and application thereof
A technology of quercetin and derivatives, which is applied in the field of compound preparation and application, can solve problems such as unseen systematic research, achieve obvious pathological hyperplasia, excellent inhibition of NRK-49F proliferation activity, and pathological hyperplasia inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
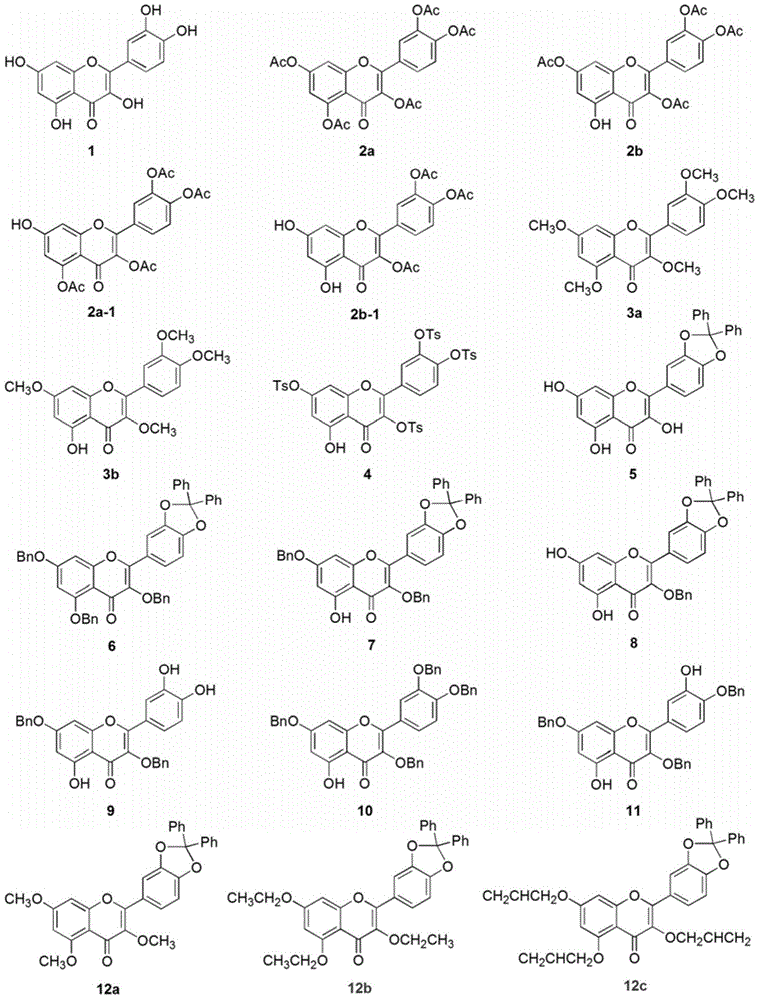
Embodiment 1 5
[0022] The synthesis of embodiment 1 five substituted quercetins and four substituted quercetins
[0023] Compound 2a: Dissolve quercetin 1 (500mg, 1.65mmol) in 15mL DMF, add acetic anhydride (1.25mL, 13.24mmol), triethylamine (2.76mL, 20mmol), stir the reaction system at room temperature for 3h, and detect the raw materials by TLC The reaction disappeared completely. The reaction solution was poured into ice water, a white solid was precipitated, the suspension was filtered to obtain a crude product, and the crude product was recrystallized with methanol to obtain a white compound 2a with a yield of 83%. Product characterization: 1 HNMR (300MHz, CDCl 3 ) δppm7.75–7.66 (m, 2H), 7.39–7.31 (m, 2H), 6.88 (d, J=1.9Hz, 1H), 2.43 (s, 3H), 2.34 (s, 6H), 2.34 (s , 6H). MS-ESI (m / z): [M+H] + : 513.10.
[0024] Compound 2b: Dissolve quercetin 1 (300mg, 1.00mmol) in 10mL DMF, add acetic anhydride (0.37mL, 4.00mmol), triethylamine (0.83mL, 6.00mmol), react the reaction system at roo...
Embodiment 2
[0030] The synthesis of embodiment 2 selective protection quercetin compounds
[0031] Compound 5: Quercetin 1 (302mg, 1.00mmol) was dissolved in 20mL of diphenyl ether, dichlorodiphenylmethane (300μL, 1.50mmol) was added, and the reaction system was stirred at 175°C for 0.5h, and the raw materials basically disappeared as detected by TLC . After the reaction solution was cooled to room temperature, a dark red solid was precipitated after adding 50 mL of petroleum ether, and the crude product was obtained by filtration. The crude product was subjected to column chromatography (PE:EtOAc=4:1) to obtain compound 5 with a yield of 86%. Product characterization: 1 HNMR (300MHz, DMSO-d 6 ) δppm 12.38 (s, 1H), 10.84 (s, 1H), 9.66 (s, 1H), 7.86–7.77 (m, 2H), 7.61–7.50 (m, 4H), 7.49–7.39 (m, 6H) , 7.20 (d, J=8.3Hz, 1H), 6.47 (d, J=2.0Hz, 1H), 6.19 (d, J=2.0Hz, 1H).
[0032] Compound 6: Compound 5 (932 mg, 2.00 mmol) was dissolved in 30 mL of acetone, and K 2 CO 3 (1.38g, 10.00mmo...
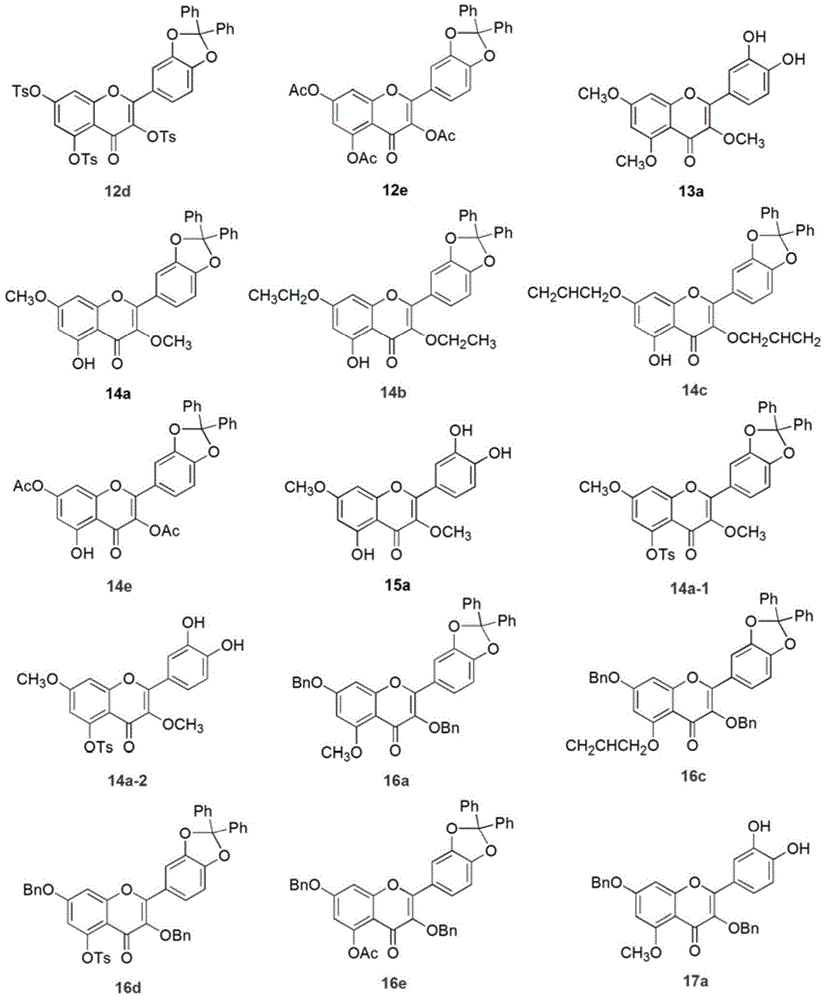
Embodiment 3
[0038] Synthesis of compound in the derivatization reaction of embodiment 3 intermediate 5
[0039] Compound 12a: Compound 5 (600 mg, 1.29 mmol) was dissolved in 20 mL of acetone, and K 2 CO 3 (1.4g, 10mmol), Me 2 SO 4 (0.61mL, 6.44mmol), the reaction system was stirred and reacted at 60°C for 3h, and TLC detected that the starting material disappeared completely. The reaction solution was filtered to remove inorganic salts, and the filtrate was spin-dried to obtain a crude product. The crude product was subjected to column chromatography (PE:EtOAc=1:2) to obtain compound 12a with a yield of 75%. Product characterization: 1 HNMR (300MHz, CDCl 3 ) δppm7.72-7.66 (m, 2H), 7.64–7.56 (m, 4H), 7.44–7.36 (m, 6H), 6.99 (d, J=8.3Hz, 1H), 6.47 (d, J=2.2Hz , 1H), 6.33 (d, J=2.2Hz, 1H), 3.95 (s, 3H), 3.88 (s, 3H), 3.88 (s, 3H).
[0040] Compound 12b: Compound 5 (200 mg, 0.43 mmol) was dissolved in 15 mL of acetone, and K 2 CO 3 (326mg, 2.36mmol), diethyl sulfate (186μL, 2.14mmol)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com