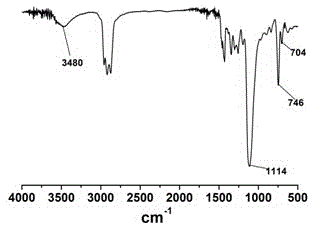
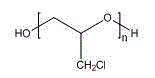
Method for preparing polyepichlorohydrin by catalyzing epichlorohydrin polymerization by using three-element iron catalyst
A polyepichlorohydrin and epichlorohydrin technology, applied in the field of polymer synthesis, can solve the problems of low activity, small molecular weight of the product, wide distribution of PDI, etc., and achieve the effects of high yield, easy reaction control and light color
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] In a 100 mL Schlenk polymerization bottle, add 20 mL of anhydrous and oxygen-free hexane and 7.8 mL of epichlorohydrin in sequence, heat in a water bath at 70 °C for 15 min, and then add 0.5 mL of ferric isooctanoate solution ( 0.1 mol / L hexane solution), 0.25 mL triethylaluminum solution (1 mol / L hexane solution), 0.5 mL diethyl phosphite solution (0.2 mol / L hexane solution). Put the polymerization bottle into a 70°C water bath and react for 2 hours. After the reaction, 5% hydrochloric acid ethanol solution was added to terminate the polymerization, the supernatant was poured out, and the obtained polymer was washed with a large amount of ethanol, dried and weighed, and the yield of the polymer was 41.0%. The polymer sample was tested by GPC, and the number average molecular weight of the polymer was 12000, and the molecular weight distribution was 1.15.
Embodiment 2
[0022] In a 100 mL Schlenk polymerization bottle, add 20 mL of anhydrous and oxygen-free hexane and 7.8 mL of epichlorohydrin in sequence, heat in a water bath at 70 °C for 15 min, and then add 0.5 mL of ferric isooctanoate solution ( 0.1 mol / L hexane solution), 1.0mL triisobutylaluminum solution (1mol / L hexane solution), 0.5mL diethyl phosphite solution (0.2 mol / L hexane solution), put the polymerization bottle Put it into a 70°C water bath and react for 2 hours. After the reaction, 5% hydrochloric acid ethanol solution was added to terminate the polymerization, the supernatant was poured out, and the obtained polymer was washed with a large amount of ethanol, dried and weighed, and the yield of the polymer was 68.0%. The polymer sample was tested by GPC, and the number average molecular weight of the polymer was 15200, and the molecular weight distribution was 1.31.
Embodiment 3
[0024] Into a 100 mL Schlenk polymerization bottle, add 20 mL of anhydrous and oxygen-free hexane and 7.8 mL of epichlorohydrin in sequence, heat in a water bath at 50 ° C for 15 min, and then add 0.5 mL of iron neodecanoate solution ( 0.1 mol / L hexane solution), 1.2 mL triisobutyl aluminum solution (1 mol / L hexane solution), 0.6 mL tributyl phosphate solution (0.2 mol / L hexane solution), put the polymerization bottle Put it into a 50°C water bath and react for 6 hours. After the reaction, 5% hydrochloric acid ethanol solution was added to terminate the polymerization, and the supernatant was poured out. The obtained polymer was washed with a large amount of ethanol, dried and weighed, and the yield of the polymer was 56.0%. The polymer sample was tested by GPC, and the number average molecular weight of the polymer was 13600, and the molecular weight distribution was 1.21.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com