Real-time fluorescent multiple PCR (Polymerase Chain Reaction) rapid detection kit for common pathogens leading to ocular infection
A real-time fluorescence and eye infection technology, applied in the field of nucleic acid detection, can solve problems affecting disease diagnosis, etc., and achieve the effect of short detection time and high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Example 1: Detection of HSV 1: A positive control of HSV 1 was used as a sample. For the acquisition of corresponding primer / probe combinations and positive controls, see Example 2. 2 μl of internal control (5 μM) was added to samples and negative control prior to extraction. Nucleic acids were extracted from samples using MagMAX Total Nucleic Acid Isolation Kit (Invitrogen). Each primer / probe combination (1.5 μl) and nucleic acid extracted from the sample or negative control (10 μl) were then added to TaqMan Fast Virus 1-Step Master Mix (Invitrogen, 13.5 μl). The final sample concentration was 5 μM and the primer / probe concentration was 0.4 μM. Subsequently, each reaction tube was subjected to a PCR reaction on an ABI 7500 (Applied Biosystems) real-time fluorescent PCR instrument according to the following procedures: 50°C for 15min (reverse transcription), 95°C for 10min (hot start); 95°C for 8s (denaturation), Maintain at 60°C for 34s (annealing / extension, collect...
Embodiment 2
[0013] Embodiment 2: A detection kit for detecting 5 kinds of pathogens of ophthalmic infection at one time
[0014] 1) Design primer / probe combination: According to the nucleic acid sequence of each pathogen, perform sequence alignment among the same species to find conserved regions, and use Beacon Designer software to design multiplex PCR primers and Taqman probes for these conserved regions. The resulting primer / probe combinations were subjected to BLAST analysis on the NCBI website to ensure no cross-reaction with other microorganisms that may be present in the sample. Finally, its performance is verified by experiments. The same method was used for the design of the internal control.
[0015] The designed primers / probes are as follows:
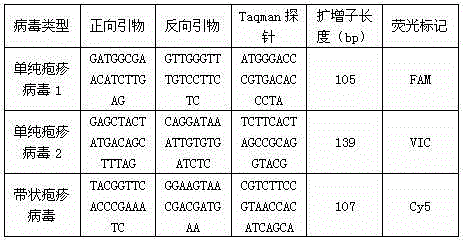
[0016] Reaction 1: Contains herpes simplex virus 1, 2 and herpes zoster virus
[0017]
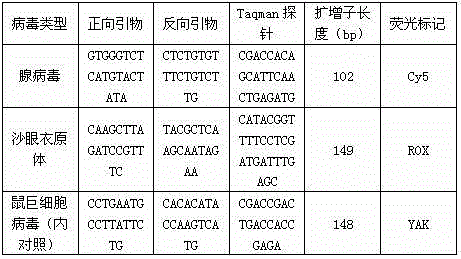
[0018] Reaction 2: Contains adenovirus, Chlamydia trachomatis and murine cytomegalovirus (internal control)
[0019]
[0020] 2) Preparati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com