Multiplex fluorescence PCR detection kit for hand-foot-and-mouth disease viruses and application thereof
A hand-foot-and-mouth disease virus and detection kit technology, applied in the field of kits, can solve the problems of time-consuming and energy-consuming, inability to effectively distinguish infections, and high detection costs, to prevent false negatives, avoid PCR reaction inhibition, and achieve good specificity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1 PCR detection kit
[0063] Described Enterovirus 71 type (EV71), Coxsackie virus A16 type (Cox A16), Dow virus universal type (EVU), internal standard gene primers and probes are as shown in Table 1, wherein in Table 1 All primers were synthesized by Sangon Bioengineering (Shanghai) Co., Ltd.;
[0064] Table 1 Enterovirus 71 (EV71), Coxsackievirus A16 (CoxA16), Dowvirus Universal (EVU), internal standard gene primers, and probes
[0065]
[0066] The kit is composed of PCR reaction buffer, enzyme system, positive control, negative control, and all primer pairs and probes in Table 1;
[0067] Table 2 is the EV71 / CA16 / EVU primer-probe mixture components (batch: 1000 parts)
[0068] serial number
raw material name
Specification
Dosage
1
EV71F
100μmol / L
50μl
2
EV71R
100μmol / L
50μl
3
EV71P
100mmol / L
25μl
4
CA16F
100μmol / L
80μl
5
CA16R
100μmol / L
80μ...
Embodiment 2
[0075] Example 2 The method for detecting Enterovirus 71 (EV71), Coxsackievirus A16 (CoxA16), and Enterovirus Universal (EVU)
[0076] (1), extraction of viral nucleic acid
[0077] Use the QIAamp MinElute Virus Spin Kit (Cat. No.: 57704) from QIAGEN or the Viral Genomic DNA / RNA Extraction Kit from Tiangen Biochemical Technology Co., Ltd. (Cat. No.: DP315) to extract nucleic acid from the sample;
[0078] (2), the configuration of the reaction system
[0079] According to the number of reaction tubes n, take PCR reaction buffer 16 μL×n, enzyme system 2 μL×n, EV71 / CA16 / EVU primer and probe mixture 2 μL×n, ensure PCR reaction buffer, EV71 / CA16 before use The mixture of / EVU primers and probes is fully dissolved and mixed, and the enzyme system is centrifuged before use to ensure that all enzymes are concentrated at the bottom.
[0080] Dispense the PCR reaction solution into PCR reaction tubes according to 20 μL / tube, and move the reaction tube containing the PCR reaction solu...
Embodiment 3
[0103] Embodiment 3 kit performance analysis
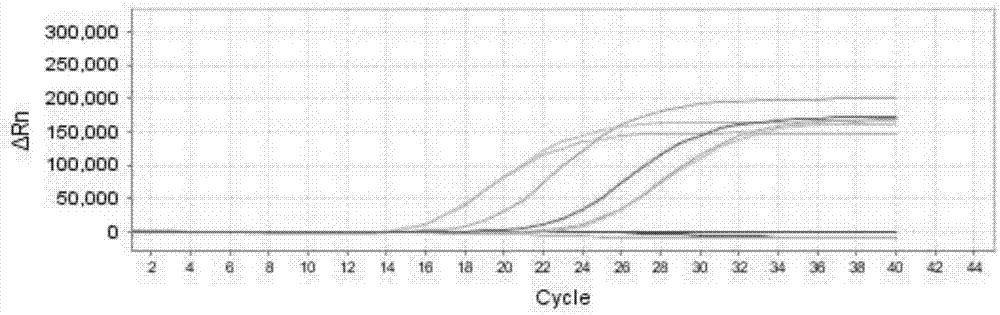
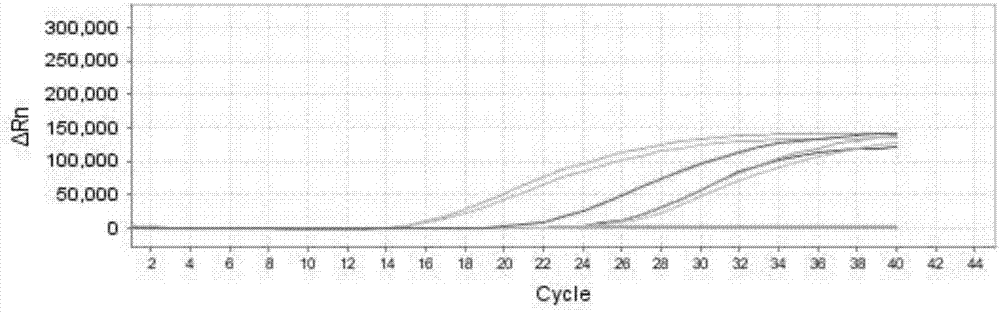
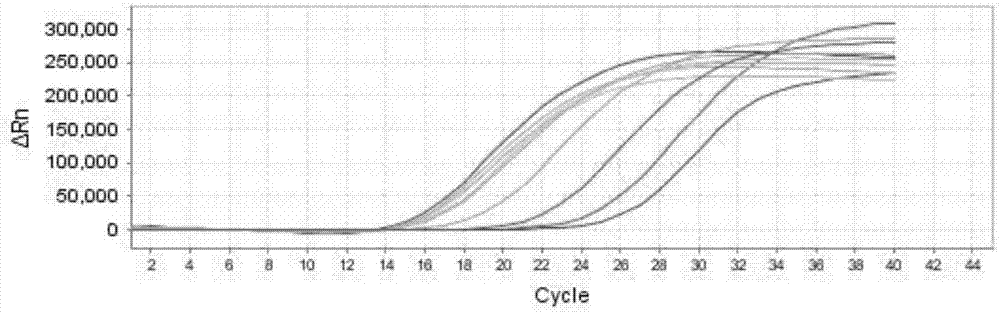
[0104] 1. Positive coincidence rate: Take the positive reference product P1-P10 as the sample to be tested, and pass the positive coincidence rate of the positive reference sample of the ABI7500 real-time fluorescent quantitative PCR instrument detection kit. The samples P1-P10 to be tested are shown in Table 8, and the test results Show, P1-P10 are all positive, coincidence rate is 100% (10 / 10), wherein, P1, P2, P7, P8, P9, P10 are EV71 positive, such as Figure 1a ; P3, P4, P7, P8, P9, P10 are negative for CA16, such as Figure 1b Shown; P1-P9 are all EVU positive, such as Figure 1c shown;
[0105] Table 8 Samples of positive reference products to be tested
[0106]
[0107]
[0108] 2. Negative coincidence rate: Take negative reference products N1-N10 as the samples to be tested, as shown in Table 9, test on the ABI7500 real-time fluorescent quantitative PCR instrument, analyze the coincidence rate of the negative refe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com