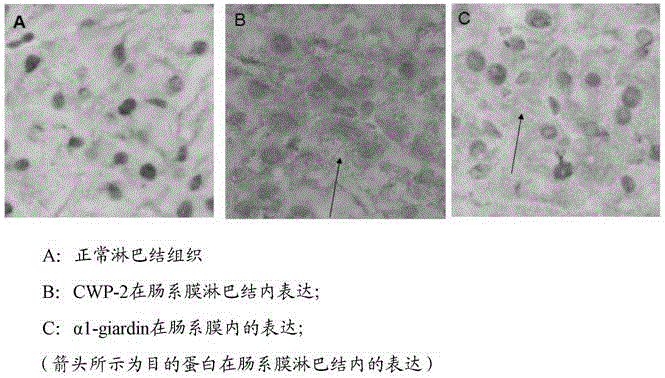
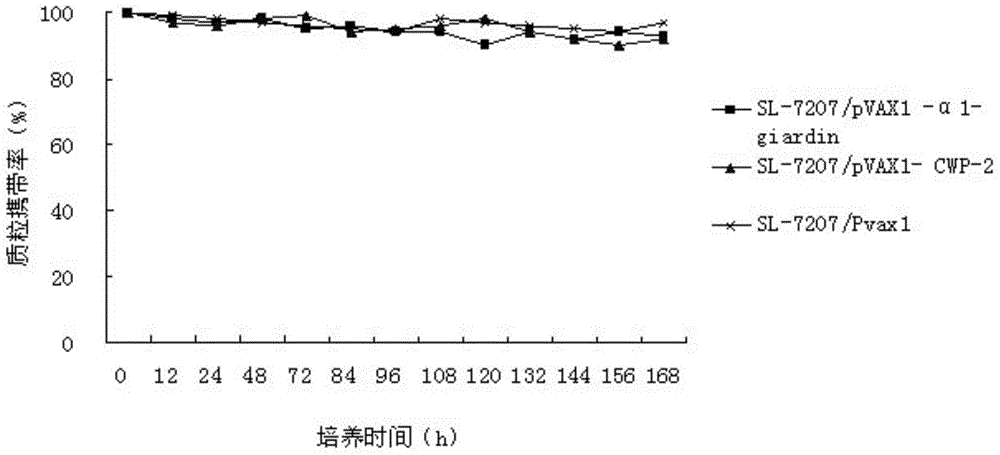
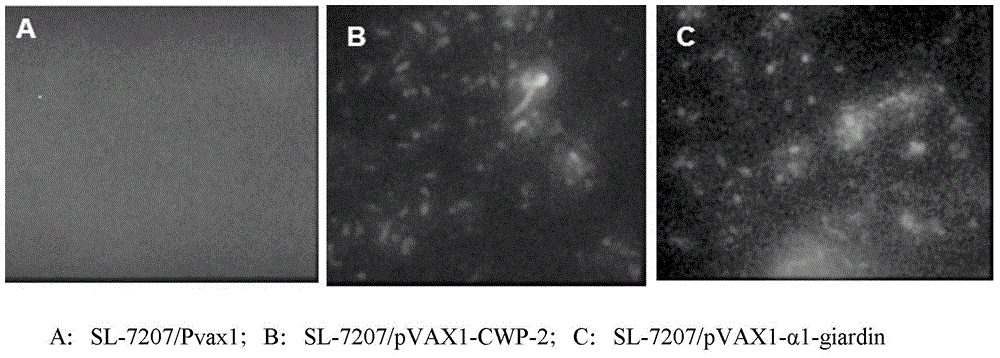
Divalent DNA vaccine for preventing giardiasis and enteric-coated preparation thereof
A DNA vaccine, giardiasis technology, applied in the field of vaccines, can solve problems such as the difficulty in ensuring the amount of animal feed, achieve the effect of easy large-scale production, overcome the single effect of vaccines, and have good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Construction and Identification of Recombinant Prokaryotic Expression Vectors pET41a-α1-giardin and pET41a-CWP-2
[0055] (1) According to the gene sequencing of α1-giardin in the Genbank database, specific primers were designed and synthesized, and HindⅢ and EcoRI restriction sites were introduced at both ends of the sequence, and the protective base GC was introduced at both ends of the restriction site.
[0056] Primers for α1-giardin:
[0057] α1-G-F1:CC AAGCTT ATGCCGAAGGTCACCGACAT(HindIII)
[0058] α1-G-R2:CG GGATCC CTTCACGCGCCAGAGGGTGC(BamHI)
[0059] (2) According to the gene sequence of Giardia CWP2 in the Genbank database, the antigenic epitope encoded by it was analyzed, and an epitope containing 125 amino acids was screened out. Design specific primers, introduce HindⅢ and EcoRI restriction sites at both ends of the sequence, and introduce protective base GC at both ends of the restriction sites.
[0060] Primers for CWP-2:
[0061] Gl-CWP2-F5:CC AAGCTT A...
Embodiment 2
[0067] Massive expression and purification of recombinant α1-giardin and CWP-2 in Escherichia coli, preparation of specific antibodies
[0068] (1) Extended expression system: Pick a single colony from the plates of pET41a-α1-giardin / BL21 and pET41a-CWP-2 / BL21 and put it into 50 ml of LB culture medium containing kanamycin. 37°C, 200rpm, shaking culture overnight; the next day, take 25ml of the overnight cultured bacterial solution and inoculate two 4L Erlenmeyer flasks containing 1L LB (containing 30μg / ml kanamycin). 37°C, 250rpm, rapid shaking culture to OD600≈0.6. Add IPTG to a final concentration of 1 mM, induce expression at 30°C, 250 rpm for 5 hours; collect the bacterial pellet by centrifugation, ultrasonically lyse, collect the supernatant (soluble expression), and use His-tag affinity chromatography column to purify the target protein.
[0069] (2) Preparation of specific polyclonal antibodies: Take 20 BALB / c female mice aged 6-8 weeks, respectively use 20 μg / 0.1ml o...
Embodiment 3
[0071] Construction of recombinant plasmids pVAX1-α1-giardin and pVAX1-CWP-2
[0072] (1) According to the gene sequencing of α1-giardin in the Genbank database, specific primers were designed and synthesized, and HindⅢ and EcoRI restriction sites were introduced at both ends of the sequence, and the protective base GC was introduced at both ends of the restriction site.
[0073] Primers for α1-giardin:
[0074] α1-G-F1:CC AAGCTT ATGCCGAAGGTCACCGACAT(HindIII)
[0075] α1-G-R2:CG GGATCC CTTCACGCGCCAGAGGGTGC(BamHI)
[0076] (2) According to the gene sequence of Giardia CWP2 in the Genbank database, the antigenic epitope encoded by it was analyzed, and an epitope containing 125 amino acids was screened out. Design specific primers, introduce HindⅢ and EcoRI restriction sites at both ends of the sequence, and introduce protective base GC at both ends of the restriction sites.
[0077] Primers for CWP-2:
[0078] Gl-CWP2-F5:CC AAGCTT AGGCAGACAGTAGTCCGC(HindIII)
[0079] Gl-CWP...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com