Method for synthesizing zinc citrate trihydrate
A technology of zinc trihydrate and synthesis method, which is applied in the separation/purification of carboxylic acid compounds, preparation of organic compounds, chemical instruments and methods, etc., and can solve the unseen synthesis process of zinc citrate trihydrate, zinc citrate There are few reports on the direct synthesis of trihydrate, etc., to achieve the effect of novel method, enhanced reaction contact, and product specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
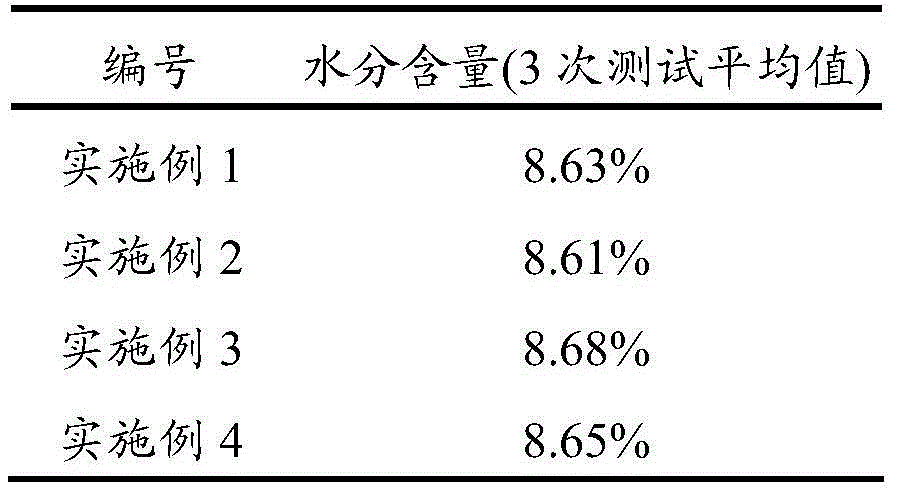
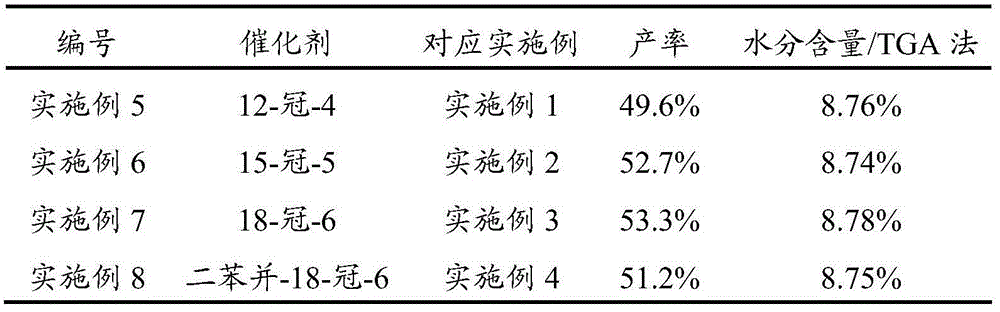
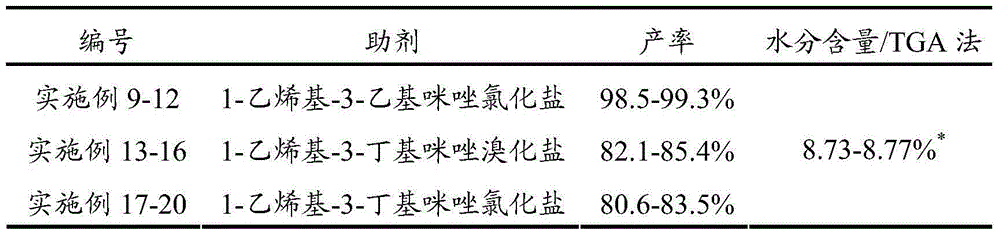
[0028] Add citric acid and deionized water into the reaction kettle, stir and heat up to 55°C, then add zinc carbonate to it, after the addition is complete, add catalyst 4-tert-butyl-calix[4]arene and additive 1-vinyl -3-Ethylimidazolium bromide, stir and heat up to 75°C, and stir and react at this temperature for 10 minutes. After the reaction is completed, centrifuge and filter, wash the obtained solid with water and absolute ethanol in sequence, and finally fully vacuum-dry. That is, zinc citrate trihydrate was obtained with a yield of 99.1% (based on citric acid).
[0029] Wherein, the mass ratio of citric acid and deionized water is 1:2, the mol ratio of citric acid and zinc carbonate is 1:3.5, and the molar dosage of 4-tert-butyl-calix[4]arene is 2% of the molar dosage of citric acid. %, the molar dosage of 1-vinyl-3-ethylimidazolium bromide is 5% of the molar dosage of citric acid. The mass spectrum data of gained zinc citrate is as follows:
[0030] MS m / z: 628.38 (...
Embodiment 2
[0032] Add citric acid and deionized water into the reactor, stir and heat up to 60°C, then add zinc nitrate into it, after the addition is complete, add catalyst 4-tert-butyl-calix[4]arene and additive 1-vinyl -3-Ethylimidazolium bromide, stirred and heated to 80°C, and stirred at this temperature for 8 minutes. After the reaction was completed, centrifuged and filtered, the obtained solid was washed with water and absolute ethanol in turn, and finally fully vacuum-dried. That is, zinc citrate trihydrate was obtained with a yield of 98.5% (calculated as citric acid).
[0033] Wherein, the mass ratio of citric acid to deionized water is 1:3.5, the molar ratio of citric acid to zinc nitrate is 1:3.7, and the molar dosage of 4-tert-butyl-calix[4]arene is 2.5% of the molar dosage of citric acid. %, the molar dosage of 1-vinyl-3-ethylimidazolium bromide is 6% of the molar dosage of citric acid. The mass spectrum data of gained zinc citrate is as follows:
[0034] MS m / z: 628.36 ...
Embodiment 3
[0036] Add citric acid and deionized water into the reactor, stir and heat up to 65°C, then add zinc carbonate to it, after the addition is complete, add catalyst 4-tert-butyl-calix[4]arene and additive 1-vinyl - 3-Ethylimidazolium Bromide, stir and heat up to 85°C, and stir and react at this temperature for 7 minutes. After the reaction is completed, centrifuge and filter, wash the obtained solid with water and absolute ethanol in sequence, and finally fully vacuum-dry. That is, zinc citrate trihydrate was obtained with a yield of 98.8% (calculated as citric acid).
[0037] Wherein, the mass ratio of citric acid and deionized water is 1:5, and the molar ratio of citric acid and zinc carbonate is 1:4, and the molar dosage of 4-tert-butyl-calix [4] arene is 3% of the molar dosage of citric acid. %, the molar dosage of 1-vinyl-3-ethylimidazolium bromide is 8% of the molar dosage of citric acid. The mass spectrum data of gained zinc citrate is as follows:
[0038] MS m / z: 628.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com