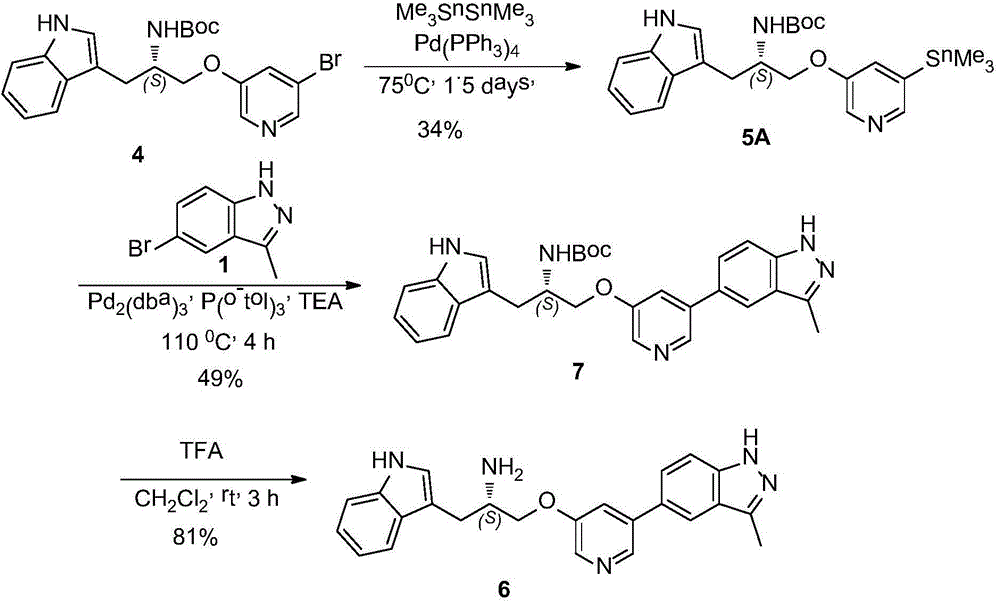
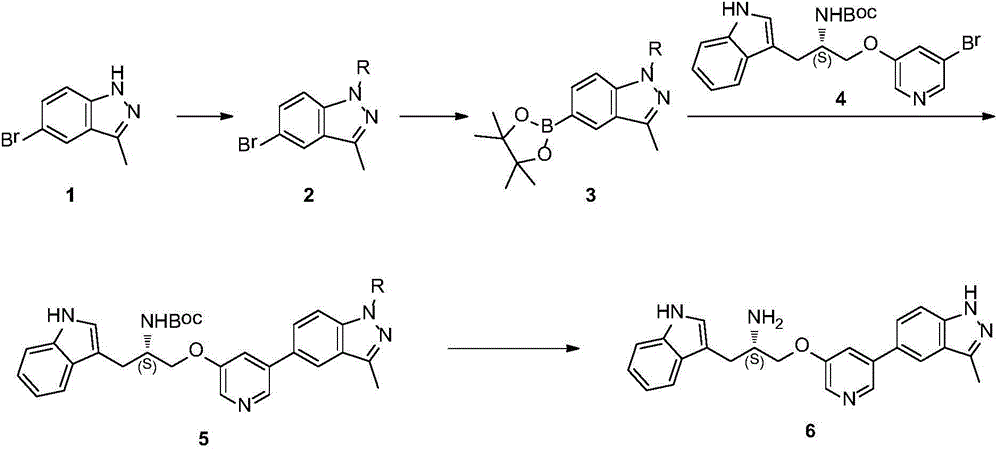
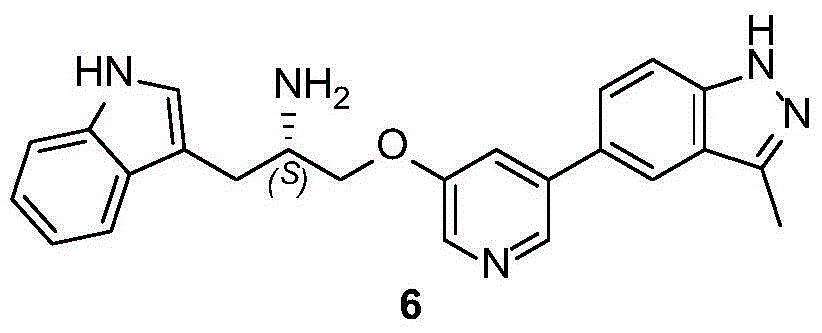
Synthesis method of ATP competitive small-molecule AKT inhibitor A443654
A synthetic method and small molecule technology, applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of low total yield, high toxicity, and low economic efficiency, and achieve the effect of shortening the reaction time and increasing the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1: the preparation of compound 2
[0055]
[0056] Compound 1 (50g, 237mmol, 1eq), 3,4-dihydropyran (60g, 713mmol, 3eq), pyridinium p-toluenesulfonate (3g, 11.8mmol, 0.05eq), chloroform (250ml , 5V), heated to reflux for 3 hours, and TLC detected that the reaction of raw materials was complete. The reaction system was lowered to room temperature, added saturated aqueous sodium bicarbonate (500ml), stirred for 5 minutes, allowed to stand, separated the organic phase, extracted the aqueous phase with chloroform (200ml) once more, combined the organic phases, and washed with saturated brine (500ml ) and water (500ml) were washed once each, dried over anhydrous sodium sulfate, and concentrated to give a black liquid (70g, Y=100%).
[0057] 1H NMR (CDCl 3 ,500MHz): δppm 7.72(s,1H),7.53(s,1H),7.29(s,1H),5.62(m,1H),4.10(m,1H),3.73(m,1H),2.63(s ,3H), 2.19(m,1H), 2.09(m,1H), 1.65(m,4H); ESI / MS: m / z=295(M+H)+.
Embodiment 2
[0058] Embodiment 2: the preparation of compound 3
[0059]
[0060] In the there-necked flask of 1L, add compound 2 (50g, 168.8mmol, 1eq), diboronic acid pinacol ester (45g, 177.3mmol, 1.05eq), potassium acetate (50g, 506.5mmol, 3eq) and DMSO (500ml), Stir for 15min under nitrogen protection, add Pd(dppf)Cl 2 (6.9g, 8.44mmol, 0.05eq), replaced with nitrogen, heated to 70°C, and reacted overnight. LCMS monitors that the reaction of the raw materials is complete, and the temperature is lowered to room temperature, water (500ml), ethyl acetate (500ml), and silica gel (50g) are added, stirred for 5 minutes, and then filtered. The organic phase was separated and the aqueous phase was extracted once more with ethyl acetate (500ml). The organic phases were combined, washed once with saturated brine, dried over anhydrous sodium sulfate, and concentrated to obtain 57.1 g of a black liquid, Y=100%). It was directly used in the next reaction without purification.
[0061] 1H NMR(...
Embodiment 3
[0062] Embodiment 3: the preparation of compound 5
[0063]
[0064] In the 3L there-necked flask, add compound 4 (150g, 336mmol, 1eq), compound 3 (138g, 403.3mmol, 1.2eq), sodium carbonate (142.5g, 1344.3mmol, 4eq), dioxane (900ml, 6V), Water (600ml, 4V), add Pd(dppf)Cl under nitrogen protection 2 (8.2g, 10.1mmol, 0.03eq), replaced with nitrogen, and then warmed up to 75 for overnight reaction. LCMS monitored the complete reaction of the starting material. Add water (1.5L), ethyl acetate (1.5L), silica gel (150g), stir for 10min, filter, the filtrate separates the organic layer, and extract the aqueous phase with ethyl acetate (1.5L) once, combine the organic phases, organic The phase was washed once with saturated brine (1.5 L), dried over anhydrous sodium sulfate, and concentrated to obtain a black oily product (143.2 g, Y=73%).
[0065] 1H NMR (CD 3 OD,500MHz):δppm 8.45(s,1H),8.25(brs,1H),7.98(s,1H),7.61(m,4H),7.37(s,1H),7.16(s,1H),7.10( m,1H),7.00(m,1H),5.80(s,1H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com