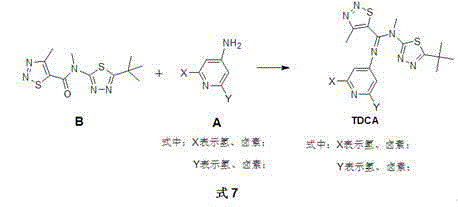
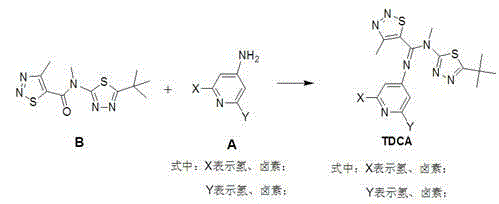
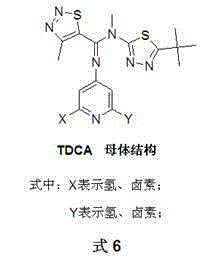
1,2,3-thiadiazole-5-formamidine compound containing three N-heterocycles and synthesis
A compound, thiadiazole technology, applied in the field of 1,2,3-thiadiazole compounds and their synthesis, can solve the problems of many by-products, low reactivity, low yield, etc., and achieve good economic benefits and social benefits benefit effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Synthesis of N-(5-tert-butyl-1,3,4-thiadiazol-2-yl)-N'-(pyridin-4-yl)-N,4-dimethyl-1, 2,3-Thiadiazole-5-carboxamidine (TDCA-1)
[0031] 1) Preparation of catalyst trimethylsilyl polyphosphate:
[0032]Under the condition of nitrogen protection, add 50ml of the dried solvent dichloromethane, phosphorus pentoxide (14.2g, 50mmol) and hexamethyldisiloxane (25.6g, 160mmol) successively into the dry three-neck reaction flask, and heat to reflux After reacting for 1 hour, the reflux reaction device was changed to a distillation reaction device, and the temperature was gradually raised to 160 ° C. During the heating process, the low boiling point solvent and unreacted hexamethyldisiloxane were distilled off, and kept at 160 ° C for 1 hour. 27.8 g of the obtained syrupy liquid (the liquid is trimethylsilyl polyphosphate), was isolated from the air, and was directly used in the next step reaction (preparation of TDCA-1) without further treatment.
[0033]
[0034]...
Embodiment 2
[0036] Example 2: Synthesis of N-(5-tert-butyl-1,3,4-thiadiazol-2-yl)-N'-(2,6-difluoropyridin-4-yl)-N,4- Dimethyl-1,2,3-thiadiazole-5-carboxamidine (TDCA-10):
[0037] 1) Preparation of the catalyst trimethylsilyl polyphosphate: The catalyst trimethylsilyl polyphosphate was prepared according to the same method as in Example 1.
[0038]
[0039] 2) Preparation of TDCA-10
[0040] Under the condition of nitrogen protection, 600ml of anhydrous sulfolane, 2,6-difluoro-4-aminopyridine (26.2g, 200mmol), compound B (N-(5-tert-butyl-1, 3,4-thiadiazol-2-yl)-N,4-dimethyl-1,2,3-thiadiazole-5-carboxamide) (59.6g, 200mmol), adding freshly distilled thionyl chloride (59.5g, 500mmol), after heating to reflux for 2 hours, the reaction was changed from a reflux device to a distillation device, and the low-boiling components in the reactor were distilled (the main component was unreacted thionyl chloride), and phosphorus pentoxide was added Solid (14.2g, 50mmol), add 27.8g of the above-m...
Embodiment 3
[0042] Synthesis of N-(5-tert-butyl-1,3,4-thiadiazol-2-yl)-N'-(2,6-difluoropyridin-4-yl)-N,4-dimethyl- 1,2,3-Thiadiazole-5-carboxamidine (TDCA-10):
[0043] According to the same method as in Example 2, the solid dosage of phosphorus pentoxide is 0g, and the other dosage and post-treatment process are unchanged to obtain the target compound N-(5-tert-butyl-1,3,4-thiadiazol-2-yl )-N'-(2,6-difluoropyridin-4-yl)-N,4-dimethyl-1,2,3-thiadiazole-5-carboxamidine ( TDCA-10 ), white solid 37.5g, liquid phase normalized content 97.9%, yield 45.9%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com