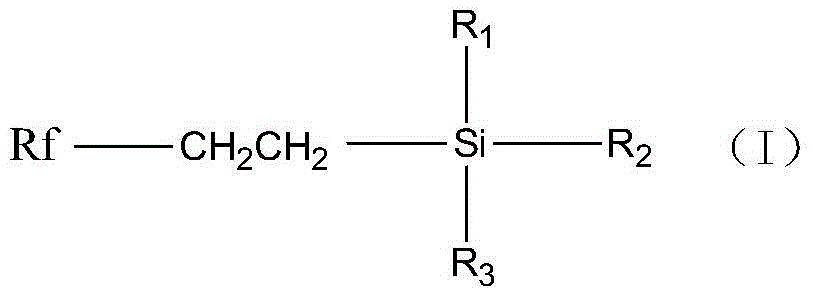
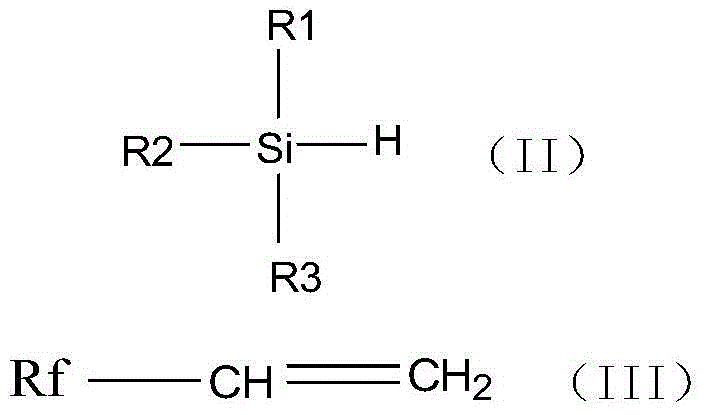
Fluoroalkyl alkoxy silane and preparation method thereof
A technology of fluoroalkylalkoxysilane and hydroalkoxysilane, which is applied in the field of organosilicon chemical synthesis, can solve the problems of three wastes, complicated processes, poor product color and luster, etc., and achieves good product quality, low corrosion, Stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 0.2g of isopropanol solution of 0.5% Pt-C complex with Pt content and 5g of trimethoxysilane into a 500ml three-necked flask with a stirring and reflux tube, heat at 80°C, and add 73g of trimethoxysilane (0.6mol) dropwise and 223g of 1H, 1H, 2H-perfluoro-1-decene (0.5mol), keep warm for 4 hours after completion, and distill to collect fractions above 100°C to obtain 256g of perfluorodecyltrimethoxysilane, based on 1H, 1H, 2H-perfluoro-1-decene The calculated yield of fluoro-1-decene is 91%.
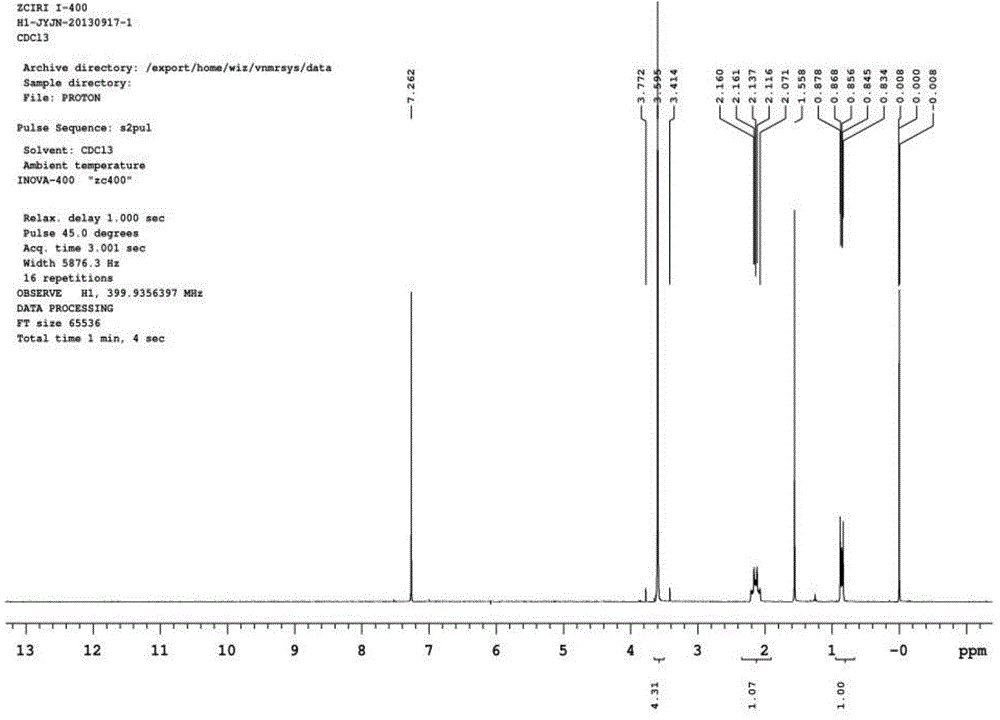
[0030] The position and assignment of the characteristic absorption peaks in the GC-MS spectrum of perfluorodecyltrimethoxysilane (m / z): 517 (0.25, M-51), 369 (0.55, CF3CF2CF2CF2CF2CF2CF2), 141 (M1, 4625), 121 (100, Si (OCH 3 ) 3 ), 91 (35.45, Si (OCH 3 ) 2 ), 59 (5.85, SiOCH3). 1H NMR spectrum see attached figure figure 1 .
Embodiment 2
[0032] According to the method of Example 1, add 0.2 g of Pt Pt-C complex isopropanol solution, 5 g of triethoxysilane, stir, heat to 100 ° C, drop 82 g of triethoxysilane (0.5 mol) from the dropping funnel and 173g 1H, 1H, 2H-perfluoro-1-octene (0.5mol), stirring for 2 hours after the dropwise addition, cooling and atmospheric pressure distillation to remove low-boiling impurities, and then vacuum distillation to collect fractions above 80°C to obtain perfluorooctane Base triethoxysilane 227g, calculated yield 85% based on trimethoxysilane.
Embodiment 3
[0034] Add the isopropanol solution of 0.4g Pt-C complex 5g methyldimethoxysilane according to the method of Example 1, stir, heat to 60 ℃, from the dropping funnel 53g methyldimethoxysilane (0.5 mol) and 223g of 1H, 1H, 2H-perfluoro-1-decene (0.5mol), after the completion of the heat preservation for 2h, distilled to collect fractions above 100°C to obtain 230g of perfluorodecylmethyldimethoxysilane, based on 1H, The calculated yield of 1H,2H-perfluoro-1-decene is 83.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com