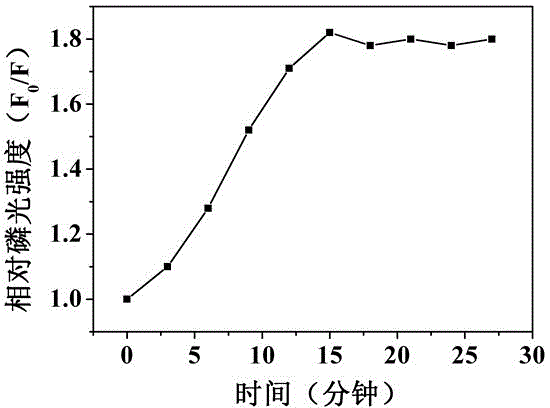
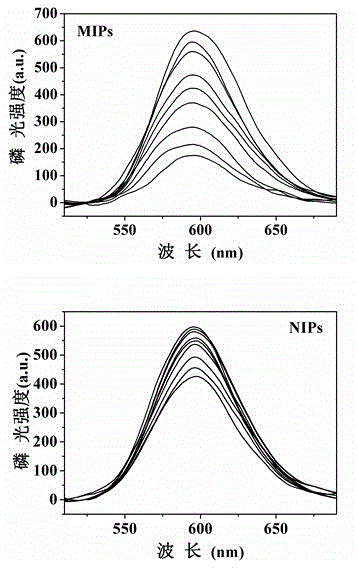
Method for preparing ZnS magnetic surface phosphorescent molecularly imprinted polymer
A technology of imprinting polymers and phosphorescent molecules, applied in chemical instruments and methods, luminescent materials, other chemical processes, etc., can solve problems such as limited selectivity, and achieve the effect of easy collection and dispersion, broad application prospects, and sensitive identification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Fe modified by KH570 3 o 4 Preparation of:
[0031] In a 100 mL beaker, add 2.0 g FeCl 3 ·6H 2 O, 5.4 g CH 3 COONa and 80 mL of ethylene glycol, after stirring and mixing evenly, the solution was transferred to a reaction kettle lined with polytetrafluoroethylene, transferred to an oven, 200 o C was reacted for 8.0 h, the reaction product was magnetically separated, washed and dried, and set aside; 100 mg of the synthesized Fe 3 o 4 , ultrasonically dispersed in a mixed solution of 50 mL of ethanol and water (volume ratio: 4:1), added 5.0 mL of ammonia water under stirring conditions, and added 1.0 mL of KH570 after stirring evenly, reacted for 5.0 h, separated the product magnetically, washed and dried Dry and set aside.
[0032] (2) Preparation of KH570-modified Mn-doped ZnS:
[0033] In a 100 mL three-neck flask, add 1.797 g ZnSO 4 ·7H 2 O, 0.1 g MnCl 2 4H 2 O, 20 mL of distilled water, the resulting mixed solution was stirred at room temperature for ...
Embodiment 2
[0041] (1) Fe modified by KH570 3 o 4 Preparation of:
[0042] In a 100 mL beaker, add 2.0 g FeCl 3 ·6H 2 O, 4.9 g CH 3 COONa and 75 mL of ethylene glycol, after stirring and mixing evenly, the solution was transferred to a reaction kettle lined with polytetrafluoroethylene, transferred to an oven, 180 o C was reacted for 7.0 h, and the reaction product was magnetically separated, and the product was Fe 3 o 4 , Wash and dry, set aside. Take 100 mg of synthetic Fe 3 o 4 , ultrasonically dispersed in a mixed solution of 45 mL of ethanol and water (volume ratio: 4:1), added 4.0 mL of ammonia water under stirring conditions, stirred evenly, added 2.0 mL of KH570, reacted for 4.0 h, separated the product magnetically, washed and dried Dry and set aside.
[0043] (2) Preparation of KH570-modified ZnS
[0044] In a 100 mL three-neck flask, add 1.797 g ZnSO 4 ·7H 2 O, 0.08 g MnCl 2 4H 2 O, 20 mL of distilled water, the resulting mixed solution was stirred at room temper...
Embodiment 3
[0052] (1) Fe modified by KH570 3 o 4 Preparation of:
[0053] In a 100 mL beaker, add 2.0 g FeCl 3 ·6H 2 O, 6.1 g CH 3 COONa and 85 mL of ethylene glycol, stir and mix evenly, transfer the solution to a reaction kettle lined with polytetrafluoroethylene, transfer to an oven, 220 o C was reacted for 9.0 h, and the reaction product was magnetically separated, and the product was Fe 3 o 4 , Wash and dry, set aside. Take 100 mg of synthetic Fe 3 o 4 , ultrasonically dispersed in a mixed solution of 55mL ethanol and water (volume ratio: 4:1), add 6.0 mL ammonia water under stirring conditions, add 3.0 mL KH570 after stirring evenly, react for 6.0 h, magnetically separate the product, wash and dry ,stand-by.
[0054] (2) Preparation of KH570-modified ZnS
[0055] In a 100 mL three-neck flask, add 1.797 g ZnSO 4 ·7H 2 O, 0.12 g MnCl 2 4H 2 O, 20 mL of distilled water, the resulting mixed solution was stirred at room temperature for 20 min under nitrogen, then 5 mL of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com