Method for improving the thermodynamic properties of metal nitrogen-based compound hydrogen storage materials
A technology of thermodynamic properties and hydrogen storage materials, applied in the production of hydrogen and other directions, can solve problems such as low entropy change, and achieve the effects of improving process, reducing cost, and shortening diffusion distance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: LiI modulation Li-Mg-N-H system thermodynamics
[0019] (1) LiNH produced by Li-Mg-N-H system 2 Add the corresponding amount of LiI, such as: 1mol4Mg(NH 2 ) 2 2mol LiNH produced in -6LiH system 2 (Reaction2), then the amount of LiI added should be 1mol, and this ratio is easy to form new product Li 3 (NH 2 ) 2 I(Reaction3).
[0020]
[0021]
[0022] (2) After adding the corresponding raw materials according to the ratio described in step (1), place them on a planetary ball mill at 100-200rpm and mix evenly.
[0023] (3) The sample mixed in step (2) is heated for dehydrogenation, and the dehydrogenation temperature is 100-250°C.
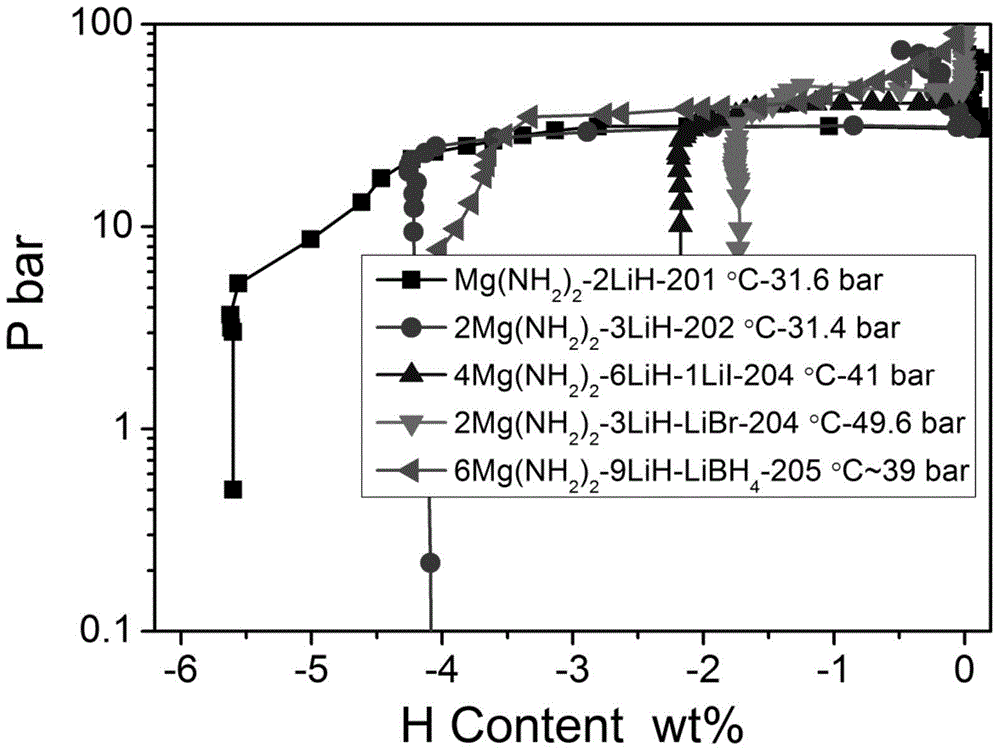
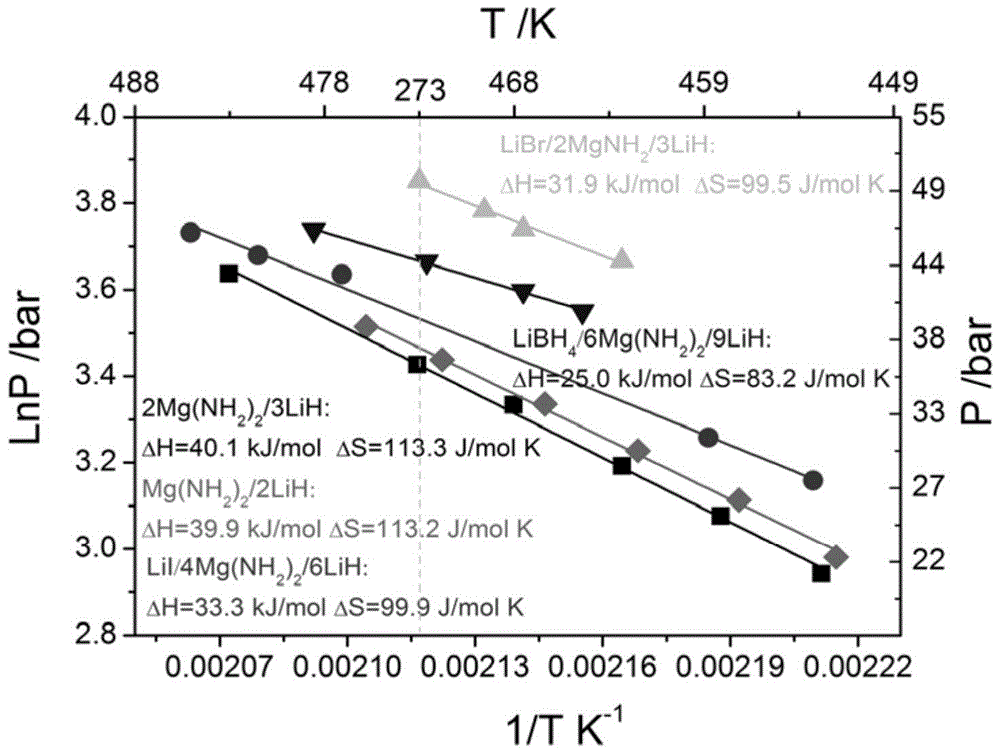
[0024] (4) obtained high platform and low dehydrogenation enthalpy value such as figure 2 with image 3 shown.
[0025] Conclusion: The addition of LiI can reduce the reaction enthalpy of Li-Mg-N-H system from 40 to 33.3 kJ / mol, which indicates that the theoretical dehydrogenation temperature of 1 atmosphere hydroge...
Embodiment 2
[0026] Example 2: LiBH 4 Modulating Thermodynamics of Li-Mg-N-H System
[0027] (1) LiNH produced by Li-Mg-N-H system 2 Add the corresponding amount of LiBH 4 , such as: 1mol6Mg(NH 2 ) 2 In -9LiH system, 3molLiNH is produced 2 (Reaction4), the added LiBH 4 The amount should be 1, 2, 3 mol, this ratio is easy to form a new product Li 4 BN 3 h 10 , Li 3 BN 2 h 8 , Li 2 BNH6 (Reaction5).
[0028]
[0029]
[0030] (2) After adding the corresponding raw materials according to the ratio described in step (1), place them on a planetary ball mill at 100-400rpm and mix evenly.
[0031] (3) The sample mixed in step (2) is heated for dehydrogenation, and the dehydrogenation temperature is 100-250°C.
[0032] (4) The obtained high dehydrogenation platform is as figure 2 shown.
[0033] Conclusion note: LiBH 4 The addition of Li-Mg-N-H system can reduce the reaction enthalpy from 40 to about 25kJ / mol, which indicates that the theoretical dehydrogenation temperature...
Embodiment 3
[0034] Example 3: LiBr modulating Li-N-H system thermodynamics
[0035] (1) According to a certain ratio of 1:1 to 1:6 (LiNH 2 :LiBr) samples were placed on a planetary ball mill at 100-400rpm and mixed evenly.
[0036] (2) Heat the sample mixed in step (1) to dehydrogenate, and the dehydrogenation temperature is 100-300°C (Reaction6&7).
[0037]
[0038]
[0039] (3) The obtained high platform such as Figure 4 shown.
[0040] Conclusion: The addition of LiBr can increase the plateau of the Li-N-H system to 43psi (280°C), which indicates that the addition of LiBr can reduce the theoretical dehydrogenation temperature of the Li-N-H system at 1 atmosphere of hydrogen pressure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com