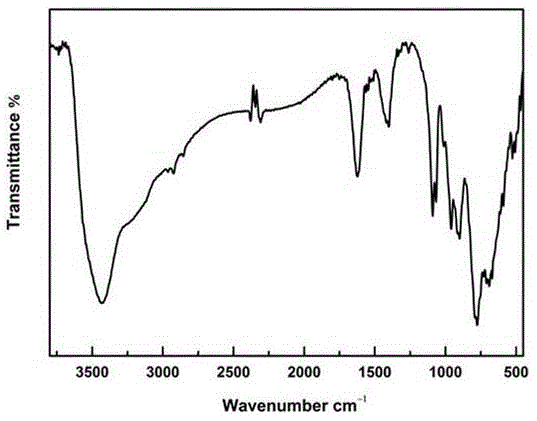
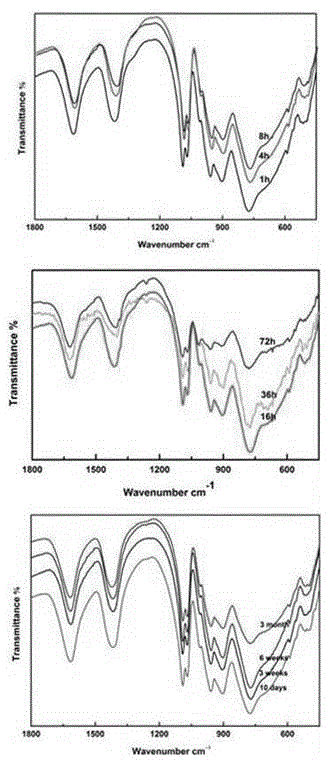
Wells-Dawson niobium-tungsten mixed polyacid rare earth derivative and preparation method and applications thereof
A technology of derivatives and rare earths, applied in tungsten compounds, chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problems of difficult derivatization and poor stability, and achieve high yield , well-structured, and easy-to-operate effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The preparation method of Wells-Dawson type niobium-tungsten mixed polyacid rare earth derivatives comprises the following steps:
[0027] (1) Synthesize K according to the literature 7 [HNb 6 o 19 ]?13H 2 For O precursors, see Filowitz M, Ho R K C, Klemperer W G, Shum W, Inorg. Chem., 1979, 18, 93–103.
[0028] (2) Synthesize K according to the literature 12 [H 2 P 2 W 12 o 48 ]?24H 2 For O precursors, see Contant R, Inorg. Synth., 1990, 27, 104–111 for literature.
[0029] (3) Under stirring conditions, mix 0.3 g K 7 [HNb 6 o 19 ]?13H 2 O (0.22 mmol), 2 ml of 1 mol / L hydrochloric acid and 0.85 g of K 12 [H 2 P 2 W 12 o 48 ]?24H 2 O (0.21 mmol) was sequentially added to the hydrogen peroxide solution (consisting of 3 ml of 30 wt% hydrogen peroxide and 30 ml of water), stirred at room temperature for 30 minutes and then added with 0.15 g of YCl 3 (0.65 mmol), adjusted the pH to 2.0 with 1 mol / L hydrochloric acid, reacted at 80°C for 8 hours, cooled ...
Embodiment 2
[0031] The preparation method of Wells-Dawson type niobium-tungsten mixed polyacid rare earth derivatives comprises the following steps:
[0032] (1) Synthesize K according to the literature 7 [HNb 6 o 19 ]?13H 2 For O precursors, see Filowitz M, Ho R K C, Klemperer W G, Shum W, Inorg. Chem., 1979, 18, 93–103.
[0033] (2) Synthesize K according to the literature 12 [H 2 P 2 W 12 o 48 ]?24H 2 For O precursors, see Contant R, Inorg. Synth., 1990, 27, 104–111 for literature.
[0034] (3) Under stirring conditions, mix 0.3 g K 7 [HNb 6 o 19 ]?13H 2 O (0.22 mmol), 2 ml of 1 mol / L hydrochloric acid and 0.85 g of K 12 [H 2 P 2 W 12 o 48 ]?24H 2O (0.21 mmol) was added successively to hydrogen peroxide solution (composed of 3 ml 30% hydrogen peroxide and 30 ml water), stirred at room temperature for 40 minutes, and then 0.18 g YCl was added. 3 (0.77 mmol), adjusted the pH to 2.0 with 1 mol / L hydrochloric acid, reacted at 85°C for 8 hours, cooled to room temperatur...
Embodiment 3
[0036] The preparation method of Wells-Dawson type niobium-tungsten mixed polyacid rare earth derivatives comprises the following steps:
[0037] (1) Synthesize K according to the literature 7 [HNb 6 o 19 ]?13H 2 For O precursors, see Filowitz M, Ho R K C, Klemperer W G, Shum W, Inorg. Chem., 1979, 18, 93–103.
[0038] (2) Synthesize K according to the literature 12 [H 2 P 2 W 12 o 48 ]?24H 2 For O precursors, see Contant R, Inorg. Synth., 1990, 27, 104–111 for literature.
[0039] (3) Under stirring conditions, mix 0.38 g K 7 [HNb 6 o 19 ]?13H 2 O (0.28 mmol), 2 ml 1 mol / L hydrochloric acid and 0.85 g K 12 [H 2 P 2 W 12 o 48 ]?24H 2 O (0.21 mmol) was added successively to hydrogen peroxide solution (composed of 3 ml 30% hydrogen peroxide and 30 ml water), stirred at room temperature for 35 minutes, and then 0.27 g YCl was added. 3 (1.16 mmol), adjusted the pH to 2.0 with 1 mol / L hydrochloric acid, reacted at 90°C for 6 hours, cooled to room temperature, f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com