Use of mir‑216 in the preparation of drugs for promoting osteogenic differentiation
A mir-216, 1.mir-216 technology, applied in the field of regenerative medicine, can solve the problems of difficult sampling, slow development of BMSC research and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: Isolation and Identification of Adult Adipose-derived Mesenchymal Stem Cells
[0019] 1. Adult adipose tissue was obtained from liposuction patients (Plastic Hospital, Chinese Academy of Medical Sciences), and informed consent was signed with voluntary donors. The donors were all healthy women aged 25-35.
[0020] 2. Isolation of hAMSCs from Adipose Tissue s The method is briefly described as follows:
[0021] The adipose tissue collected by liposuction was washed with D-Hanks to remove blood cells and anesthetics, digested with 0.2g / 100ml type II collagenase (purchased from: Life Technologies, USA) for 1 hour, and then washed twice with D-Hanks to remove collagenase .
[0022] The cells were collected by centrifugation, and the cells were separated by 2×10 6 / ml density inoculated with 58% (v / v) DMEM / F12+40% (v / v) MCDB-201, 2% (v / v) fetal bovine serum, 10ng / ml EGF, 10ng / ml PDGF, 1 × insulin-transferrin-selenite (ITS), l × linoleic acid-bovine serum album...
Embodiment 2
[0034] Example 2: miRNA s transfection
[0035] 1. Reagents:
[0036] (1) The transfection reagent is Lipofectamine2000 (Invitrogen);
[0037] (2) miRNA to be transfected s :
[0038] miR-216 powder was synthesized by American Life Technologies;
[0039] An irrelevant sequence (SEQ ID No. 2: UUCUUCGAACGUGUCACGUTT) with a similar length to miR-216 was synthesized using known techniques as an NC control.
[0040] 2. miRNA to be transfected s Stock solution configuration:
[0041] will contain the miRNA to be transfected s Centrifuge the tube at low speed for 5 minutes, add Opti-MEM to dissolve miRNA according to the instructions of Lipofectamine2000 (Invitrogen) s , vortexed and centrifuged briefly to prepare a 20 μM stock solution.
[0042] 3. Cell preparation:
[0043] Take the hAMSCs in the logarithmic growth phase of the third passage, which are 70%-80% confluent s Perform transfection.
[0044] 4. Transfection process:
[0045] Dilute the miRNA to be transfected ...
Embodiment 3
[0051] Example 3: hAMSCs differentiate into osteoblasts
[0052] The hAMSCs of the third generation (not transfected), the cells obtained in Example 2 (transfected with miR-216, or transfected with NC control) were divided into 2×10 4 cells / cm 2 The density was inoculated in T25 culture flasks, and after adhering to the wall overnight, when the cells reached 70%-80% confluence, the osteogenic induction medium was replaced (10% FBS, 10nM dexamethasone, 0.2mM ascorbic acid were added to H-DMEM medium) and 10mM beta-sodium glycerophosphate) to continue culturing, and the medium was changed every two days and a half.
[0053] Samples were collected on days 0, 3, 6, and 9 after induction, and the expression changes of osteogenic phenotype marker genes were detected by qRT-PCR.
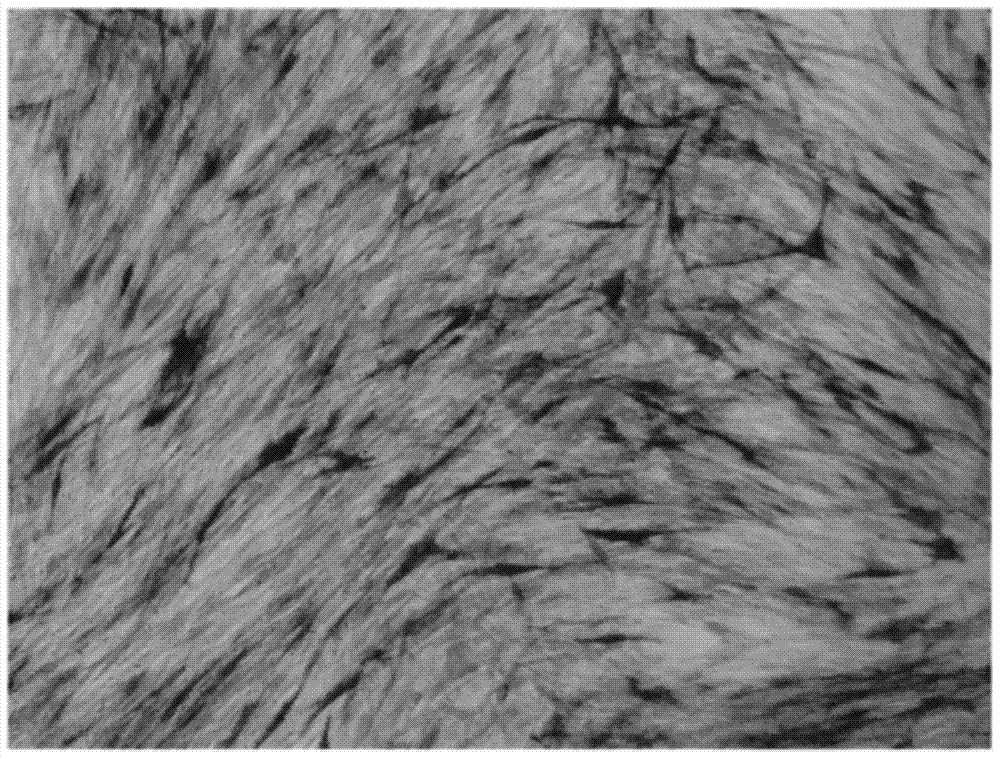
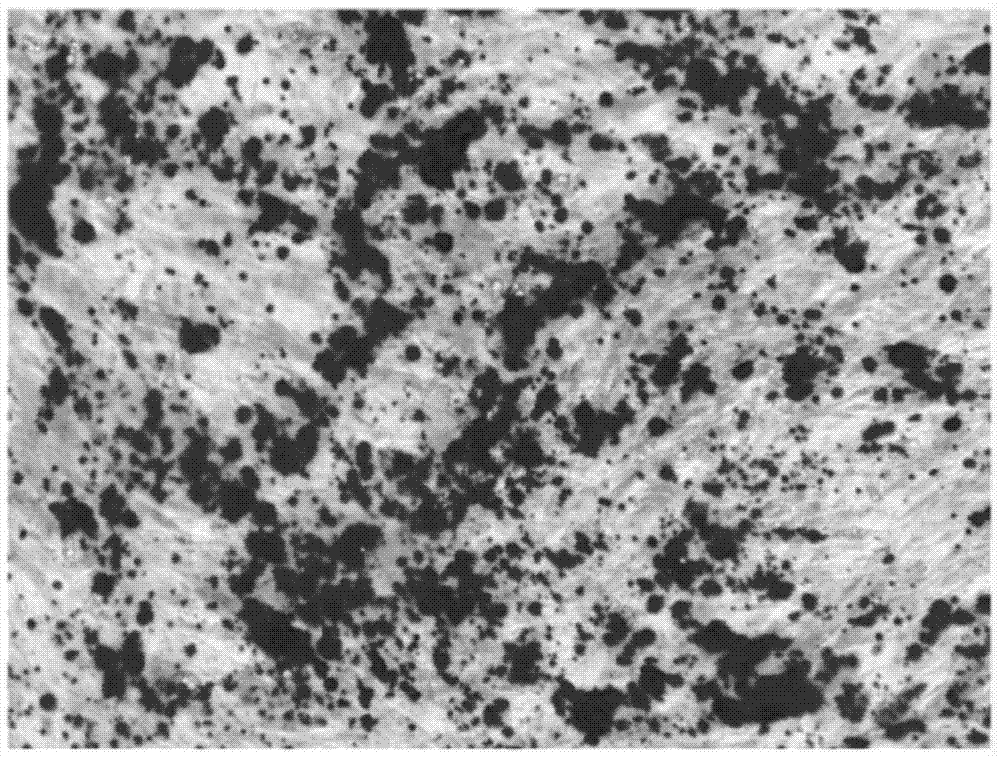
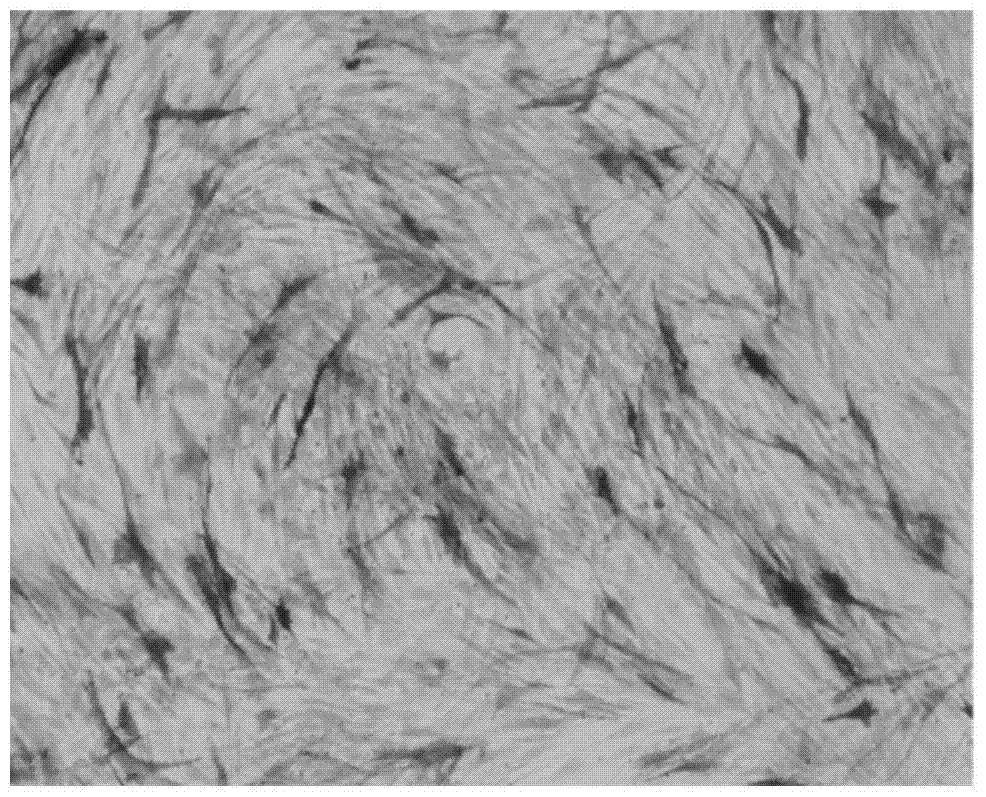
[0054] Calcification and mineralized matrix production were identified by alkaline phosphatase staining and alizarin red staining at 3 days and 12 days after induction, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com