Radionuclide labelled specific sentinel node photographic developer and preparation method thereof
A technology of sentinel lymph node and imaging agent, applied in directions such as radioactive carriers, can solve the problems of poor stability of the medicine box and no inert gas protection of the medicine box, and achieve the effects of low cost, good clinical application prospect and convenient use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The preparation of embodiment 1 specific sentinel lymph node imaging agent precursor (1)
[0027] According to the mass molar ratio of rituximab and HYNIC bifunctional chelating agent of 1:20, mix at 25°C in the dark for 15 minutes, and separate and collect with PD-10 column after the reaction is completed.
Embodiment 2
[0028] The preparation of embodiment 2 specific sentinel lymph node imaging agent precursor (2)
[0029] Mix rituximab with HYNIC bifunctional chelating agent or 2-IT or mercaptoethanol at a ratio of 1:3, and react in the dark for 30 minutes at 4°C; after the reaction is completed, use a PD-10 column to separate, purify, and collect.
Embodiment 3
[0030] The preparation of embodiment 3 specific sentinel lymph node imaging agent kit (1)
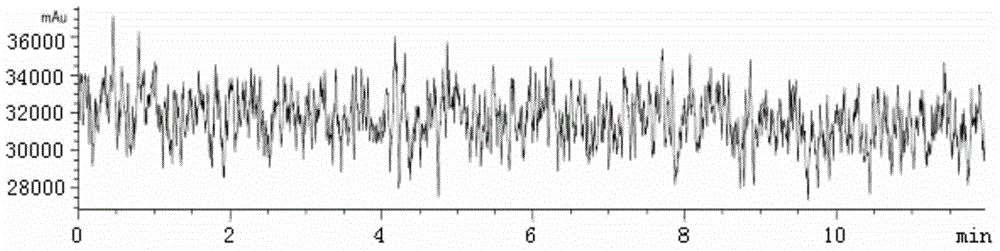
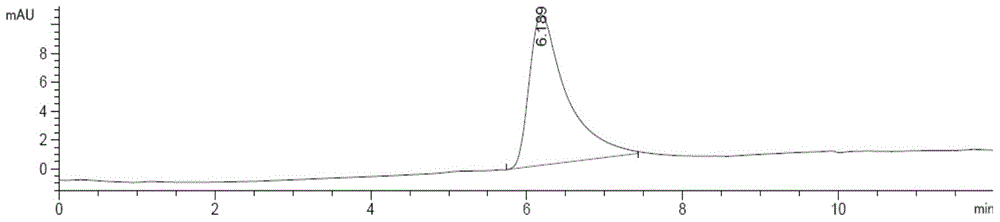
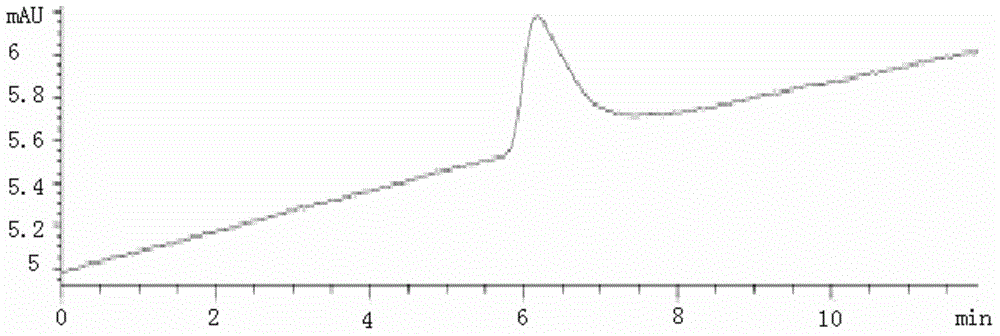
[0031] 1. Subpackage the isolated monoclonal antibody, add 10 mg of fructose excipient according to 1.0 mg of antibody under sterile conditions, and freeze-dry for 10 h at 1 Pa and -100 °C in a freeze dryer; 2. After the freeze-drying is completed, the system is slowly charged with helium to standard atmospheric pressure to protect the system with inert gas. Then, the SLN-F kit is obtained by packaging under the condition of aseptic and below -20°C. Add 0.3mL PBS to the kit to dissolve, and perform Radio-HPLC analysis. The analysis results are shown in Figure 1A with Figure 1B , no radiochemicals were present in the radioactive detector ADC1. The corresponding retention time Rt = 6.19 min at VWD 280 nm. It shows that the monoclonal antibody maintains the original molecular weight.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com