Recombinant human-derived collagen product for skin barrier function or haemorrhoids and preparation method
A technology of human collagen and skin barrier, applied in bandages, absorbent pads, medical science, etc., can solve problems such as lack of internal hemorrhoid products, skin barrier function damage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
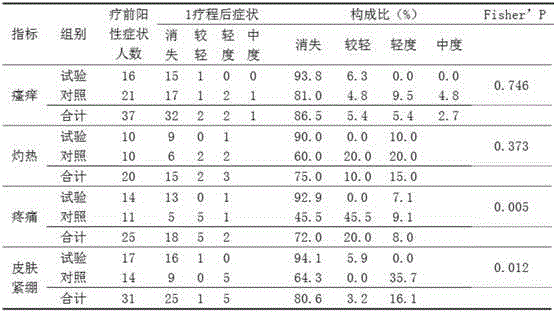
[0176] Medical skin collagen repair function dressing, ingredients include recombinant human collagen 0.05%; moisturizing emollient: sodium hyaluronate 0.8%, glycerin 0.3%, vaseline 0.2%, hydrogenated lecithin 0.15%, polydimethylsiloxane 0.8%, xanthan gum 0.5%, butanediol 0.2%, white mineral oil 0.8%, dipropylene glycol 0.3%, sunflower oil 3.5%, vitamin E 0.1%, vitamin C 0.5%; co-solvent: stearic acid 0.2%, 18 alcohol 0.3%; antibacterial preservatives: phenoxyethanol 0.8%, sodium methylparaben 0.05%, sodium propylparaben 1%; the balance water.
[0177] Taking the preparation of 1kg of dressing as an example, the concentration of recombinant human collagen is 0.05%, that is, each 1kg of dressing contains 0.5g of recombinant human collagen, and other ingredients are also calculated based on this. The specific preparation method is to dissolve the moisturizing emollient and co-solvent into 850g of pure water, stir evenly, then add recombinant human collagen filtered through a 0.2...
Embodiment 2
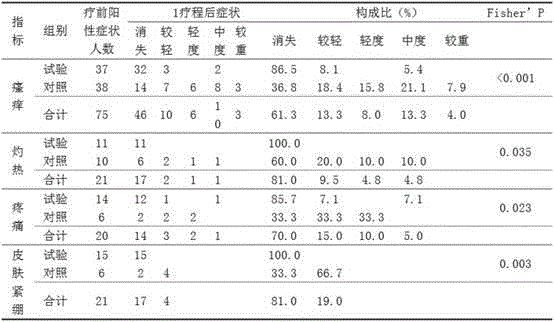
[0179] Medical skin collagen repair function dressing, ingredients include recombinant human collagen 0.1%; moisturizing emollient: sodium hyaluronate 0.1%, glycerin 3%, petrolatum 2%, hydrogenated lecithin 1.5%, polydimethylsiloxane 1.5%, xanthan gum 0.25%, butanediol 2%, white mineral oil 5%, dipropylene glycol 3%, sunflower oil 6%, vitamin E 0.75%, vitamin C 0.35%; solubilizer: stearic acid 2%, 18 alcohol 1.5%; antibacterial preservatives: phenoxyethanol 0.1%, sodium methylparaben 0.12%, sodium propylparaben 0.08%; the balance water.
[0180] Taking the preparation of 1kg of dressing as an example, the concentration of recombinant human collagen is 0.1%, that is, every 1kg of dressing contains 1g of recombinant human collagen, and other ingredients are also calculated based on this. The specific preparation method is to dissolve the moisturizing emollient and co-solvent into 880g of pure water, stir evenly, then add recombinant human collagen filtered through a 0.22μm membr...
Embodiment 3
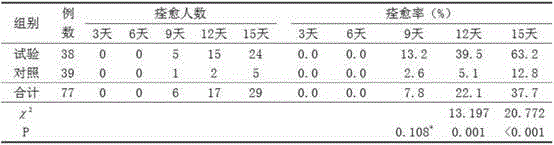
[0182] Medical skin collagen repair function dressing, ingredients include recombinant human collagen 1%; moisturizing emollient: sodium hyaluronate 1%, glycerin 0.6%, petrolatum 4%, hydrogenated lecithin 1%, polydimethylsiloxane 4%, Xanthan Gum 1%, Butylene Glycol 4%, White Mineral Oil 10%, Dipropylene Glycol 6%, Sunflower Oil 1.5%, Vitamin E 1%, Vitamin C 1%; Solvent: Stearic Acid 1%, 18 Alcohol 6%; antibacterial preservatives: phenoxyethanol 1%, sodium methylparaben 2.2%, sodium propylparaben 2%; the rest water.
[0183] Taking the preparation of 1kg of dressing as an example, the concentration of recombinant human collagen is 1%, that is, every 1kg of dressing contains 10g of recombinant human collagen, and other ingredients are also calculated based on this. The specific preparation method is to dissolve the moisturizing emollient and co-solvent into 900g of pure water, stir evenly, then add recombinant human collagen filtered through a 0.22μm membrane, continue stirring ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com