Semisynthesis method of 17-hydroxyjolkinolide B
A semi-synthetic technology of euphorbia lactone, applied in the direction of organic chemistry and the like, can solve the problem that the yield is only 7-16%, and achieve the effects of reducing the treatment cost, increasing the market supply and reducing the production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
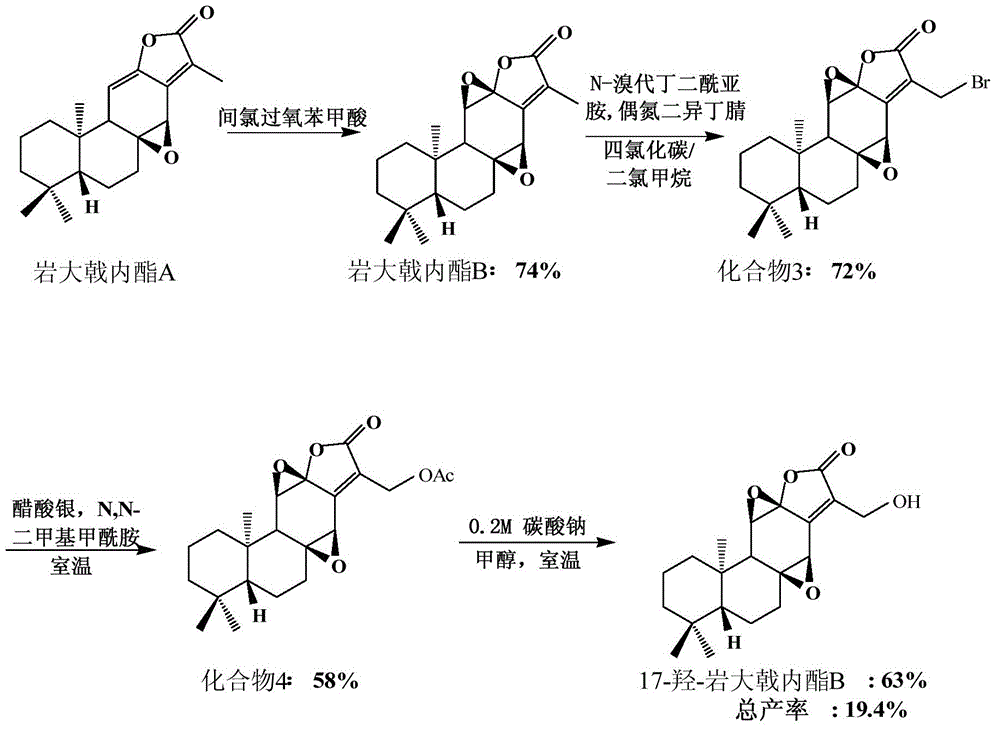
[0037] Embodiment one, figure 1 It is the semi-synthetic route 1 diagram of 17-hydroxy-petrolactone B (17-hydroxyjolkinolide B) of the present invention, and the reaction steps are as follows figure 1 Shown:
[0038] Preparation of Jolkinolide B (Compound 2)
[0039] image 3 It is the semi-synthetic route figure of rock euphorbia lactone B (Jolkinolide B) of the present invention, as image 3 As shown, dissolving Jolkinolide A (204.0mg, 0.6mmol) in dichloromethane (200.0mL) to form a 0.03mol / L solution, adding m-CPBA (206.0mg, 2.0eq ), stirring at room temperature, after reacting for 4h, adding 10% Na 2 S 2 o 3 Aqueous solution (100 mL) quenched the reaction. It was extracted with ethyl acetate (3×100 mL), and the ethyl acetate layer was washed with saturated brine (200 mL), dried over anhydrous sodium sulfate, filtered and concentrated to obtain 312.0 mg of a crude product. The product was separated by silica gel column chromatography and eluted with Hexane / EtOAc=8 / 2 (...
Embodiment 2
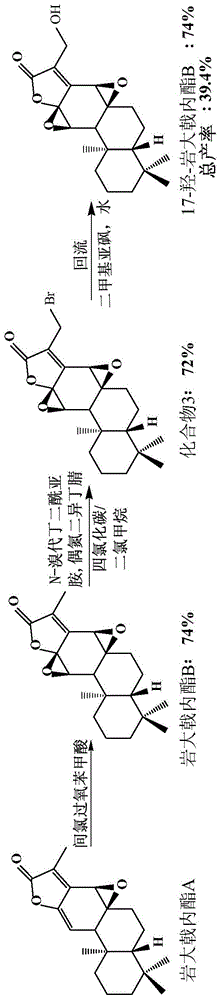
[0046] Embodiment two, figure 2 It is the semi-synthetic route 2 figure of 17-hydroxyl-petrolactone B (17-hydroxyjolkinolide B) of the present invention, and the reaction steps are as follows figure 2 Shown:
[0047] Compound 3 (22.1 mg, 0.06 mmol) was dissolved in DMSO (2.5 mL), and water (0.3 mL) was added, and stirred at room temperature. After reacting for 72h, add saturated NaHCO 3 Aqueous solution (10 mL) quenched the reaction. with CHCl 3 (10 mL×4) extraction. The organic phases were combined, washed with saturated brine (30.0 mL×4), and dried over anhydrous sodium sulfate. Filtration and concentration under reduced pressure gave 20.3 mg of crude product. The product was separated and purified by column chromatography, Hexane / EtOAc=6 / 4 (100mL) was eluted, and the target product 17-hydroxyjolkinolide B (17-hydroxyjolkinolide B) (16.5mg) was obtained, and the yield was 74 %.
[0048] Figure 4 It is the compound Jolkinolide A (Jolkinolide A) 1 H NMR diagram, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com