Photopolymerizable composition, photopolymerizable inkjet ink, and ink cartridge
一种光聚合性、组合物的技术,应用在油墨、涂层、印刷等方向,能够解决困难等问题,达到外观改善的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
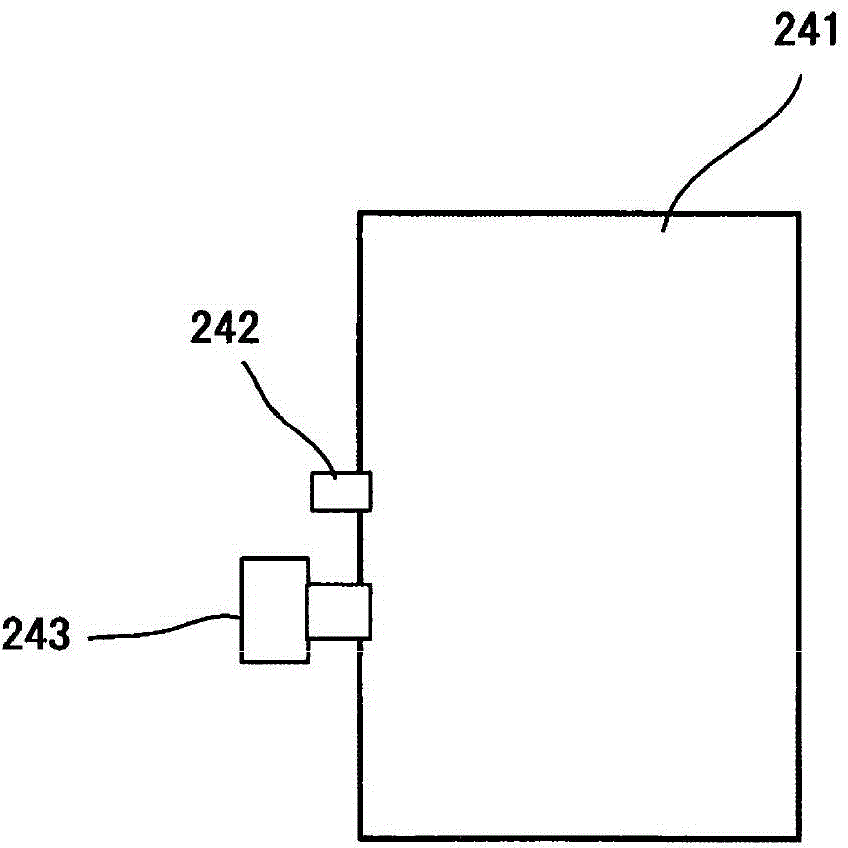
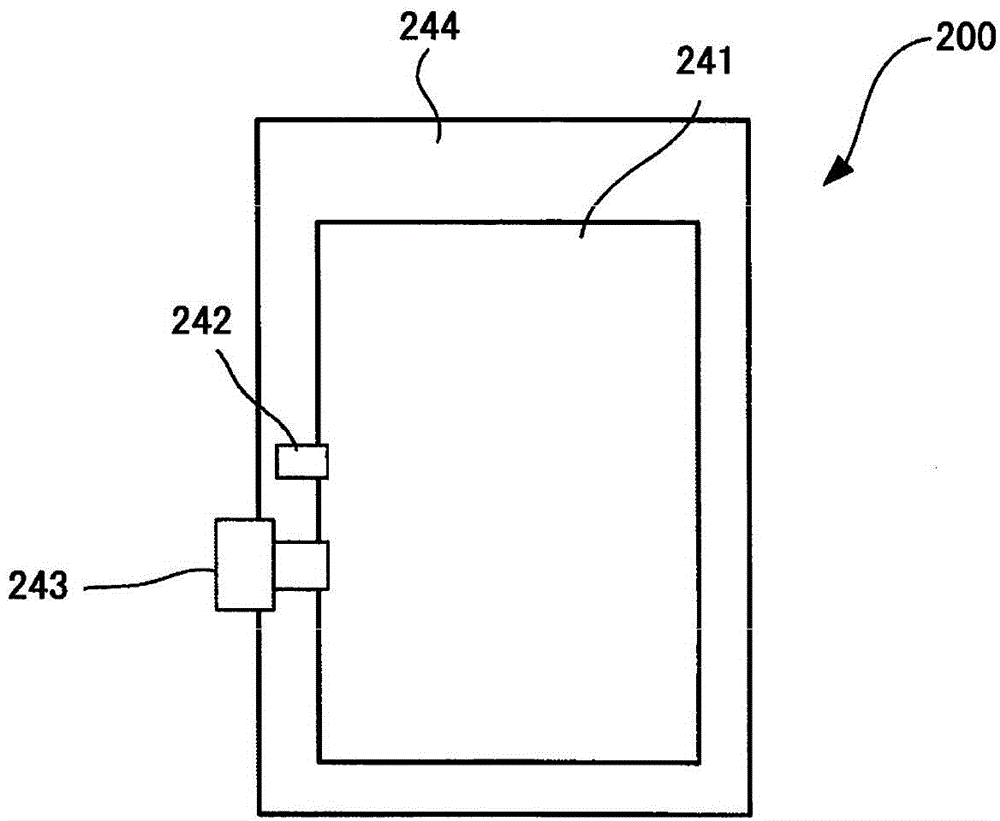
Image
Examples
Embodiment 1
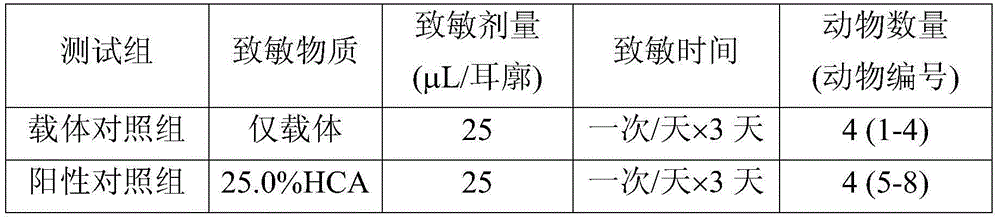
[0128] The ink of Example 1 having a viscosity of 8 mPa·s at 25° C. was obtained by mixing the following materials (a) to (d) with the mixing ratio presented in Table 3-1.
[0129] a: (meth)acrylate
[0130] a1: Diethylene glycol dimethacrylate 95
[0131] (2G(1.1), manufactured by Shin-Nakamura Chemical Co., Ltd.)
[0132] a2: Dipentaerythritol hexaacrylate 5
[0133] (TMPT-3EO(3.1), manufactured by Shin-Nakamura Chemical Co., Ltd.)
[0134] a3: Ethylene oxide modified trimethylolpropane trimethacrylate 0
[0135] b: polymerization initiator
[0136] b1: 1-hydroxycyclohexyl phenyl ketone 20
[0137] c: polyether modified polysiloxane compound
[0138] c1: (CH 3 )Si-O-[Si(CH 3 ) 2 -O] a -[Si(CH 3 )(X)-O] b -Si(CH 3 ) 3 0.1
[0139] d: (meth)acrylate other than (a) 0
[0140] (a) (Meth)acrylates negative for skin sensitization
[0141] (b) Photoradical polymerization initiator
[0142] (c) Polyether-modified polysiloxane ...
Embodiment 2 to 8, comparative Embodiment 1 to 2
[0194] Each of the inks of Examples 2 to 8 and the inks of Comparative Examples 1 to 2 was similarly obtained by mixing the materials of (a) to (d) at the mixing ratios presented in Table 3-1.
[0195] Each of the obtained inks was subjected to measurement of viscosity (mPa·s) at 25°C, 45°C and 60°C, and its leveling degree indicating the smoothness of the coating film was evaluated in the same manner as in Example 1. The results are presented in Table 3-1.
Embodiment 9 to 16
[0196] [Examples 9 to 16, Comparative Examples 3 to 4]
[0197] Each of the inks of Examples 9 to 16 and the inks of Comparative Examples 3 to 4 was similarly prepared by mixing the materials of (a) to (d) at the mixing ratios presented in Table 3-2 (numerical unit: parts by mass ) to obtain by mixing.
[0198] Each of the obtained inks was subjected to measurement of viscosity (mPa·s) at 25°C, 45°C and 60°C, and its leveling degree indicating the smoothness of the coating film was evaluated in the same manner as in Example 1. The results are presented in Table 3-2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com