Compound, as well as preparation method and application thereof
A technology of compounds and concentrates, applied in the field of compounds and their preparation, can solve problems such as systematic research that has not been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
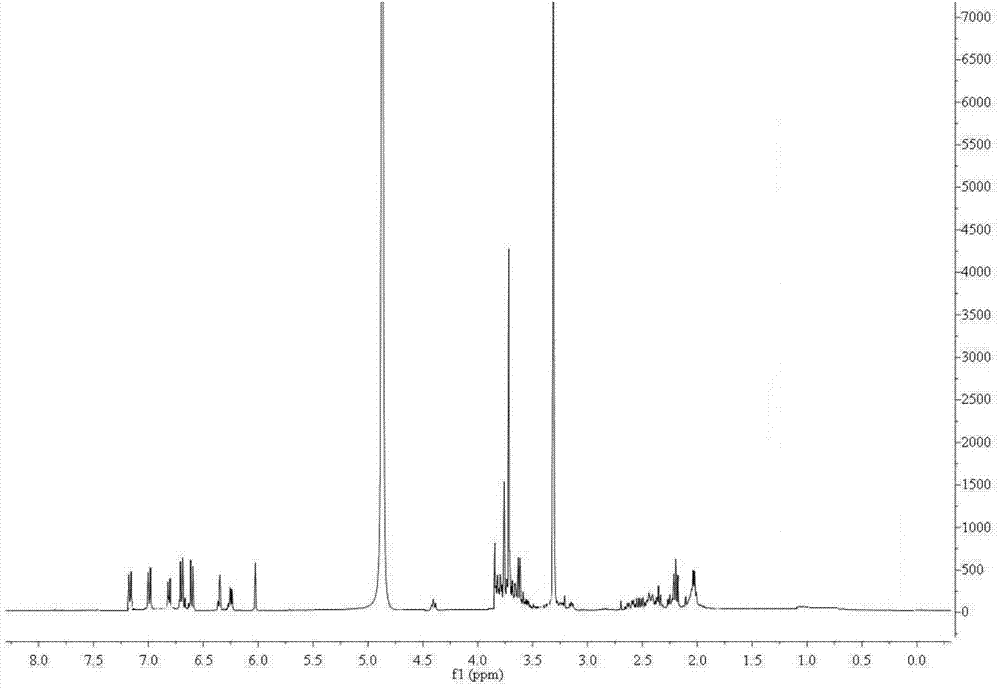
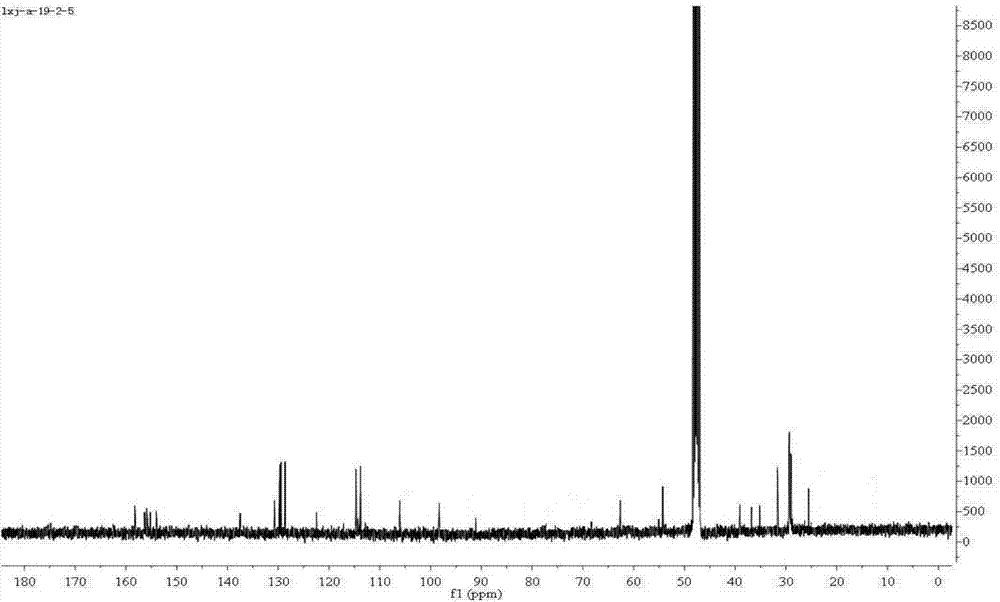
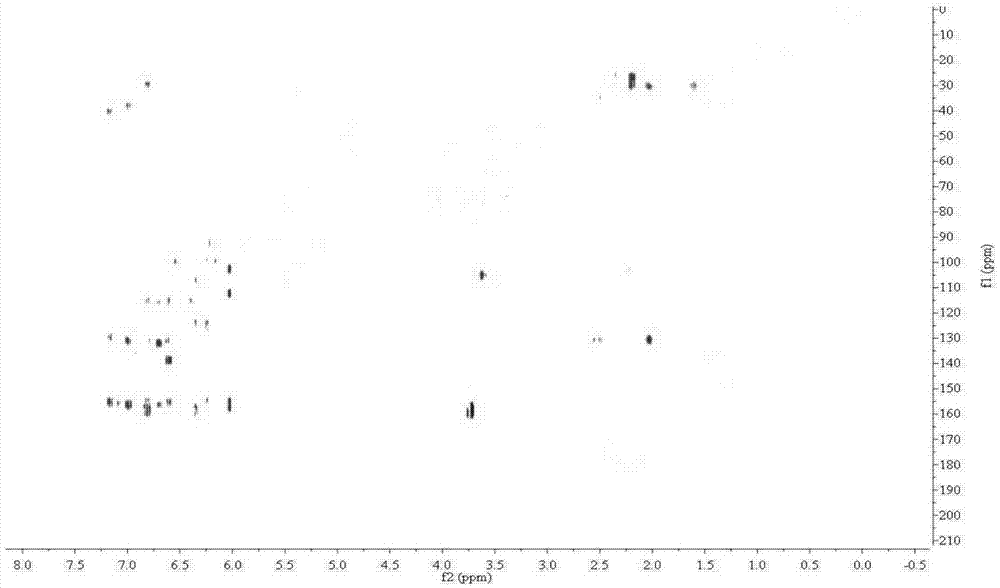
Image
Examples
preparation example Construction
[0039] The present invention also provides a preparation method of a compound of formula (I), comprising:
[0040] 1) Carry out chromatographic separation of dragon's blood through a macroporous adsorption resin column, elute with an aqueous solution of alcohol of the first mass concentration, and an aqueous solution of alcohol of the second mass concentration, and collect the eluted part of the aqueous solution of alcohol of the second mass concentration eluent,
[0041] The alcohol is C1~C6 alcohol,
[0042] The aqueous alcohol solution of the first mass concentration is an aqueous alcohol solution of 20% to 60%,
[0043] The aqueous alcohol solution of the second mass concentration is an aqueous alcohol solution of 80% to 98%;
[0044] 2) concentrating the eluate obtained in step 1) to obtain the concentrate of the elution site of the second mass concentration of the aqueous solution of alcohol;
[0045] 3) The condensate obtained in step 2) is subjected to chromatograph...
Embodiment 1
[0068] 10g of Longxue Tongluo Capsule bulk drug was dissolved in 40% ethanol and sampled, separated by HP-20 macroporous adsorption resin chromatographic column, eluted successively with 2L 40% alcohol aqueous solution, 2L 95% alcohol aqueous solution, and collected 95 The obtained eluate was eluted with an aqueous solution of % alcohol, and the recovered eluate was concentrated to dryness to obtain a concentrate (Extract I).
[0069] Dissolve the extract I with a small amount of methanol for LH-20 gel separation, the eluent is methanol, automatically receive 1 part (2mL) every three minutes, collect 30 components in total, combine components 11-14 and record them as group Fraction A was concentrated to dryness.
[0070] Component A was dissolved in a small amount of methanol and then separated by LH-20 gel again. The eluent was methanol, and 1 part (2 mL) was automatically received every three minutes. A total of 30 components were collected, and each component A-1~A was conc...
Embodiment 2
[0075] 10g of total phenols of dragon's blood were dissolved in 40% ethanol, separated by HP-20 macroporous adsorption resin column, followed by 1.5L 20% methanol water solution, 1.5L 40% methanol water solution, 1.5L 60% methanol water solution, 1L 90 % methanol aqueous solution for elution, collect the eluate obtained from 90% alcohol aqueous solution elution, recover the eluate and concentrate to dryness to obtain a concentrate (Extract I).
[0076] Dissolve the extract I in a small amount of methanol for H separation on silica gel, and use 500 mL of a chloroform:methanol volume ratio of 15:1 solution, 500 mL of a 10:1 solution, 500 mL of a 5:1 solution, and 300 mL of a 2:1 solution as eluents. TLC spot plate UV showed that the developer was chloroform:methanol 5:1, collected and concentrated into five components. Recorded as FrI-1, FrI-2, FrI-3, FrI-4, FrI-5.
[0077] Dissolve the concentrated FrI-4 with a small amount of methanol for H separation on silica gel. The eluen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com