Recombinant vector, recombinant baculovirus prepared with the same and application of virus in preparation of malaria vaccines
A technology of recombinant baculovirus and recombinant vector, which is applied in the field of biomedical technology and can solve the problems of high cost, low expression amount, unsuitable for production needs and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
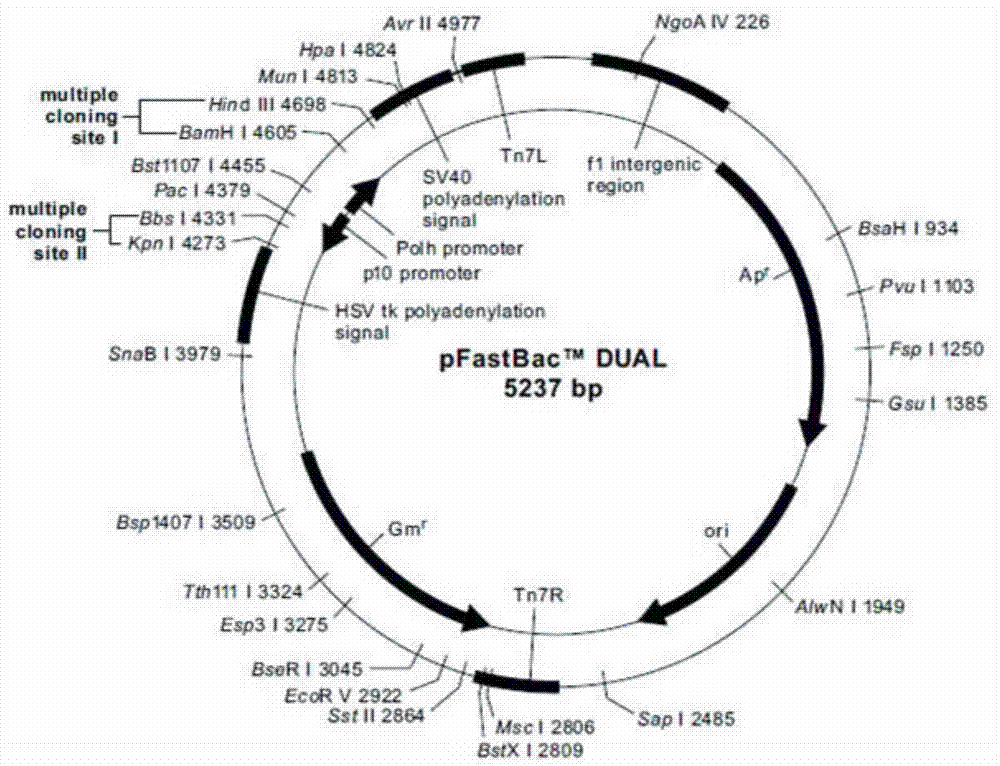
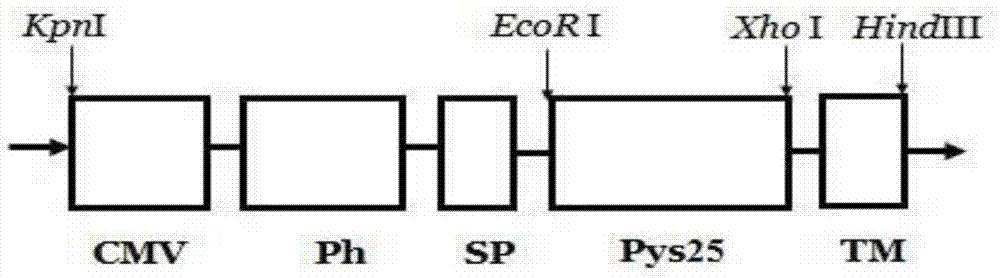
[0052] Example 1: Construction of recombinant vector pFastBacDual-CMV-Ph-SP-TM
[0053] According to the known sequences of CMV, Ph, SP, and TM, an EcoRI restriction site (GAATTC) and an Xho I restriction site (CTCGAG) were added between the SP and TM nucleotide sequences, and a KpnI restriction site was added before CMV-F Point, add HindIII restriction site after TM-R, synthesize CMV-Ph-SP-TM sequence (as shown in SEQ ID NO: 1), and design primer CMV-F (as shown in SEQ ID NO: 2), TM-R (shown in SEQ ID NO: 3). Primers are as follows:
[0054] CMV-F5'-CCC GGTACC TAGTTATTAATAG-3'
[0055] TM-R5'-CCC AAGCTT TTAATATTGTCTAC-3'
[0056] Wherein the underline is the restriction site.
[0057] Use the synthesized CMV-Ph-SP-TM sequence as a template, and use CMV-F and TM-R as upstream and downstream primers to amplify the target fragment by PCR. The PCR reaction system is 50μL, and the specific components are: 10×PCRBuffer5μL, 2.5mmol / 5 μL of dNTPs in mL, 1 μL each of CMV-F a...
Embodiment 2
[0060] Example 2: Construction of the recombinant transposable plasmid pFstBacDual-CMV-Ph-SP-Pys25-TM
[0061] Use the Pys25 target gene (as shown in SEQ ID NO: 4) as a template, and use Pys25-F (as shown in SEQ ID NO: 5) and Pys25-R (as shown in SEQ ID NO: 6) as upstream and downstream primers for PCR Amplify the target gene Pys25.
[0062] Pys25-F5'-CG GAATTC ATGAACACATACTAC-3'
[0063] Pys25-R5'-G GAATTC ATGTTGAGCTTCTTTGGC-3'
[0064] Wherein the underline is the restriction site.
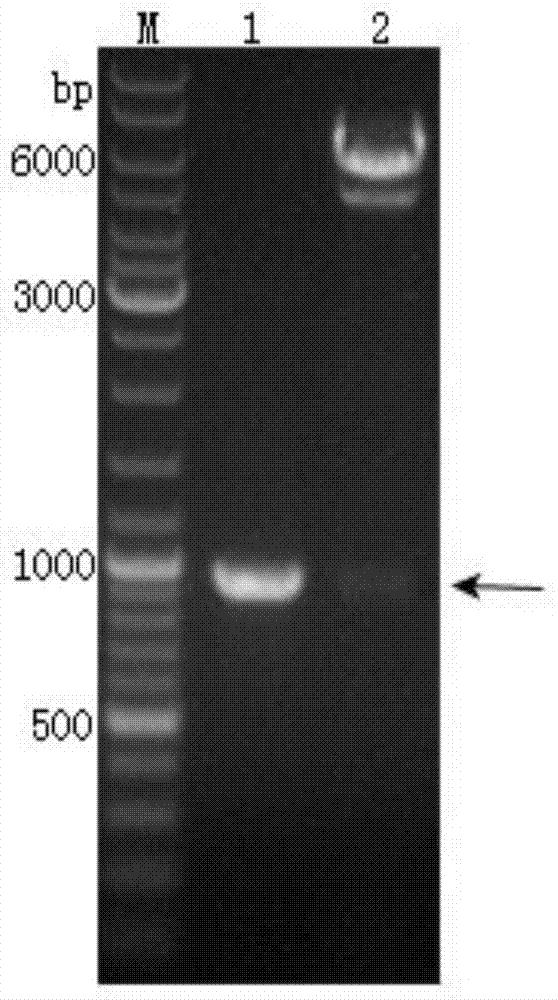
[0065] The PCR reaction system is 50 μL, and the specific components are: 10×PCRBuffer 5 μL, 2.5 mmol / mL dNTPs 5 μL, 0.01 nmol / μL Pys25-F and Pys25-R 1 μL each, template 2 μL, TaqDNA polymerase 2 μL, ddH 2 O34 μL. After each component was mixed, put it into a PCR machine. The PCR reaction parameters were: 95°C pre-denaturation for 5 minutes, 95°C denaturation for 1 minute, 53°C annealing for 30 seconds, 72°C extension for 45 seconds, 30 cycles, and 72°C extension for 10 minutes. After t...
Embodiment 3
[0067] Embodiment 3: the acquisition of Bombyx mori recombinant baculovirus BmPys25
[0068] The recombinant transposable plasmid pFastBacDual-CMV-Ph-SP-Pys25-TM, which was successfully identified for recombination, was transformed into Escherichia coli DH10Bac competent cells containing the baculovirus shuttle vector Bacmid, in the presence of kanamycin, gentamicin, tetracycline, X-gal and IPTG were cultured on the LB culture plate (operated according to the instructions), and the blue and white spots were screened after homologous recombination by transposition. After 48 hours of dark culture, the white spots were picked, and the white spots continued to be treated with tetracycline and kanamycin. , Gentamicin, X-gal and IPTG in the LB culture solution of 48h after shake culture, extract recombinant baculovirus genomic DNA with isopropanol, use M13 universal primer (M13-F as shown in SEQ ID NO: 7 , M13-R as shown in SEQ ID NO: 8), Pys25-F and Pys25-R identified the insertion...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com