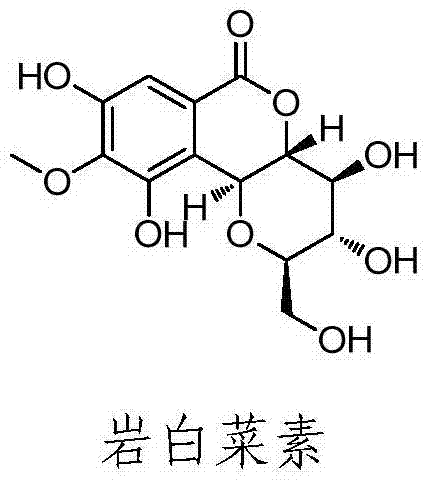
Bergenin derivative as well as preparation method and application thereof
A technology of petracenin and derivatives, which is applied in the field of preparation and derivatives of petracenin, which can solve the problems of large drug resistance of toxic and side effects, and drugs can only alleviate the disease, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
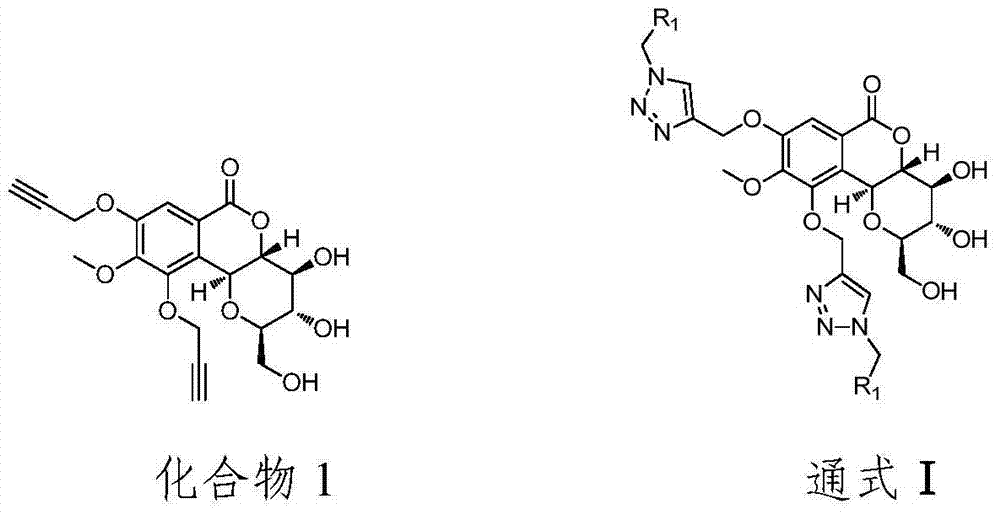
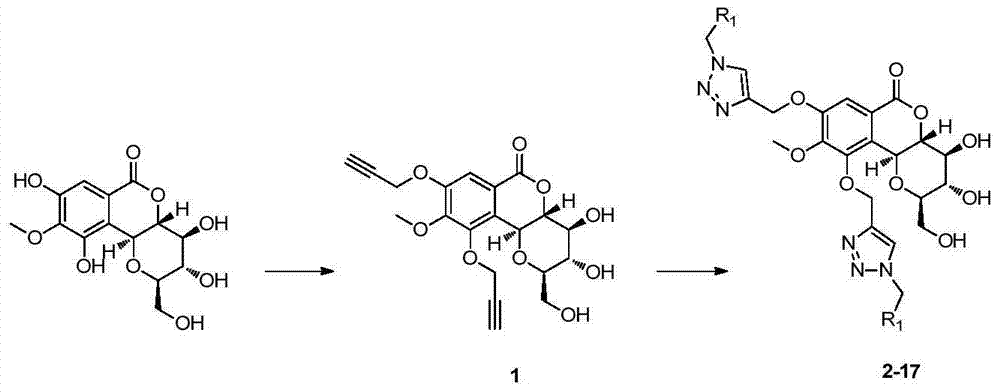
[0048] Embodiment 1: the preparation of compound 1
[0049] (1) Take 1mmol petracenin and 3mmol KI, 2mmolK 2 CO 3 In the reaction solvent 3mL NMP, stir at room temperature until the sample is completely dissolved, then add 3mmol 3-bromopropyne, react at 60°C for 4h, and detect by TLC, the reaction is complete.
[0050] (2) After directly adding 15 mL of ethyl acetate and 15 mL of distilled water to the reaction system obtained in (1) for extraction, the organic layer was washed with 10 mL of saturated brine. Anhydrous NaSO for organic phase 4 Dry the powder for 15-30 minutes.
[0051] (3) The product obtained in step (2) is filtered, concentrated, separated and purified by silica gel column chromatography, and eluted with petroleum ether / ethyl acetate 1 / 1 (V / V), and the eluted part is concentrated to obtain a single white powder target product. The yield was 66.7%.
[0052] through 1 H NMR detection, the structural characterization of compound 1 is as follows:
[0053]...
Embodiment 2
[0055] Embodiment 2: the preparation of compound 2
[0056] 1. Preparation of azide compounds
[0057] Take 1 mmol of benzyl bromide and 2 mmol of sodium azide in a 5 mL dry round bottom flask, add 2 mL of DMF, and react at 30° C. for 10 h, and TLC detects that the reaction is complete. Add 10 mL of ether and 10 mL of distilled water to the reaction solution for extraction, wash the organic phase with 6 mL of saturated brine, and wash the organic layer with anhydrous NaSO 4 Dry for 15 to 30 minutes, filter, and concentrate to obtain benzyl-substituted azide compounds.
[0058] 2. Preparation of Compound 2 by Click Chemistry Principle
[0059] Take 0.1mmol compound 1 (40.2mg) and 0.25mmol benzyl-substituted azide in a 25mL dry round bottom flask, add 5mg of CuSO 4 ·5H 2 O, 5 mg sodium ascorbic acid in 3 mL THF-H 2 O(V / V=1 / 1) or 3mL t-BuOH-H 2 The 1,3-dipolar cycloaddition reaction was carried out in O (V / V=1 / 1) solvent at room temperature to generate compound 2 with a yie...
Embodiment 3
[0063] Embodiment 3: the preparation of compound 3
[0064] The difference between the preparation method of compound 3 and the preparation method of compound 2 is that 4-methylbenzyl azide is prepared by reacting 4-methylbenzyl chloride with sodium azide. The yield of compound 3 was 88.8% when using click chemistry principle.
[0065] through 1 H NMR detection, the structural characterization of compound 3 is as follows:
[0066] 1 H NMR (400MHz, CD 3 OD)δ8.02(s,1H),7.94(s,1H),7.51(s,1H),7.15(dd,J=14.2,10.1Hz,8H),5.51(s,2H),5.47(s, 2H), 5.15(d, J=11.5Hz, 1H), 5.05(d, J=11.5Hz, 1H), 4.46(d, J=10.3Hz, 1H), 3.88-3.77(m, 2H), 3.76( s,3H),3.68(dd,J=11.4,6.6Hz,1H),3.39(dd,J=18.1,9.8Hz,1H),3.29(s,2H),2.28(s,6H).
[0067] The analysis of the above nuclear magnetic detection data shows that the target product 3 is generated.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com