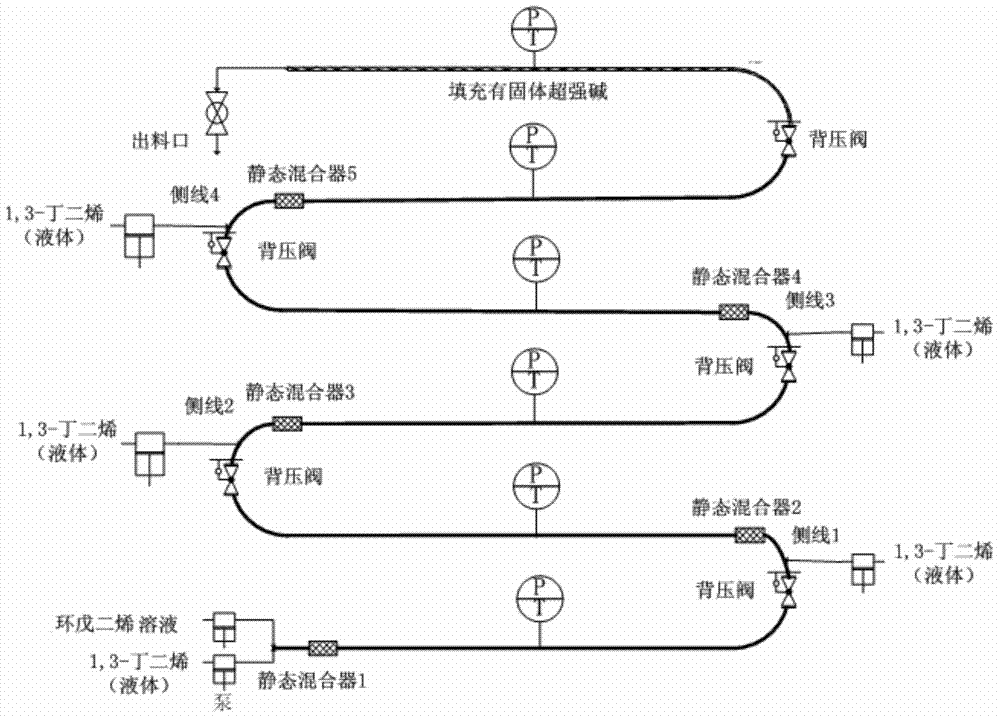
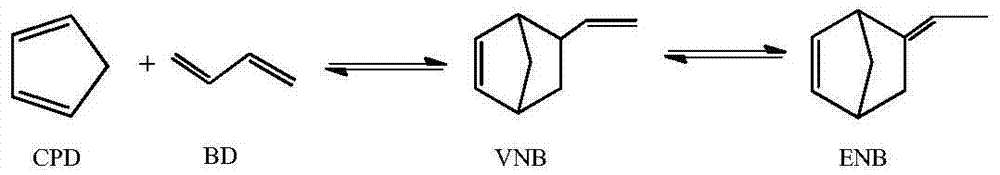
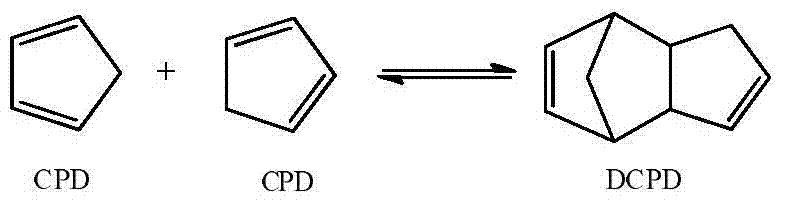
Method for synthesizing ethylidene norbornene by virtue of multi-lateral-line pressure-variable tubular reactor
A technology of ethylidene norbornene and tubular reactor, which is applied in the direction of isomerization hydrocarbon production, organic chemistry, etc., can solve the problems of many side reactions, improve safety and synthesis efficiency, facilitate sealing, and avoid reaction hot spots the effect of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] A cyclopentadiene toluene solution with a mass fraction of 60 wt % was prepared, wherein the mass ratio of cyclopentadiene to dicyclopentadiene was 19:1. That is, the cyclopentadiene toluene solution is composed of cyclopentadiene and dicyclopentadiene with a mass total concentration of 60%, and toluene as a balance (that is, a mass concentration of 40%), wherein cyclopentadiene and dicyclopentadiene The mass ratio of pentadiene is 19:1.
[0045] Under normal temperature conditions, the cyclopentadiene toluene solution is transported from the initial feed port to the tubular reaction device, and 1,3-butadiene is fed from the initial feed port, side line 1 feed port and side line 2 feed port It is divided into three equal parts and transported to the tubular reaction device, the total mass flow rate of 1,3-butadiene is 82.0g / min, the mass flow rate of cyclopentadiene toluene solution is 840.0g / min, 1,3-butadiene and The molar ratio of the cyclopentadiene toluene solutio...
Embodiment 2
[0048] Prepare a cyclopentadiene solution with a mass fraction of 100 wt%, wherein the mass ratio of cyclopentadiene to dicyclopentadiene is 4:1. That is, the cyclopentadiene solution is entirely composed of cyclopentadiene and dicyclopentadiene, wherein the mass ratio of cyclopentadiene to dicyclopentadiene is 4:1.
[0049] Under normal temperature conditions, the cyclopentadiene solution is transported from the initial feed port to the tubular reaction device, and 1,3-butadiene is distributed from the initial feed port, side line 1 feed port and side line 2 feed port. Three equal quantities are delivered to the tubular reaction device, the total mass flow rate of 1,3-butadiene is 23.4g / min, the mass flow rate of cyclopentadiene solution is 286.0g / min, 1,3-butadiene and cyclopentadiene The molar ratio of the pentadiene solution is 0.1. The two streams of 1,3-butadiene and cyclopentadiene solution are mixed by a static mixer and then enter the reaction section to react in the ...
Embodiment 3
[0051] A cyclopentadiene toluene solution with a mass fraction of 30 wt % was prepared, wherein the mass ratio of cyclopentadiene to dicyclopentadiene was 1.5:1. That is, the cyclopentadiene toluene solution is composed of cyclopentadiene and dicyclopentadiene with a total mass concentration of 30%, and toluene as a balance (that is, a mass concentration of 70%), wherein cyclopentadiene and dicyclopentadiene The mass ratio of pentadiene is 1.5:1.
[0052] Under normal temperature conditions, the cyclopentadiene toluene solution is transported from the initial feed port to the tubular reaction device, and 1,3-butadiene is fed from the initial feed port, side line 1 feed port and side line 2 feed port It is divided into three equal parts and transported to the tubular reaction device. The total mass flow rate of 1,3-butadiene is 13.0g / min, the mass flow rate of cyclopentadiene toluene solution is 120.0g / min, and the mass flow rate of 1,3-butadiene The molar ratio to the cyclope...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com