A kind of preparation method of homopiperazine
A technology of homopiperazine and tosyl homopiperazine, which is applied in the field of medicine, can solve the problems of low purity of the final product, harsh reaction conditions, complicated synthesis process, etc., to suppress the formation of linear by-products and simplify the production process , the effect of improving yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
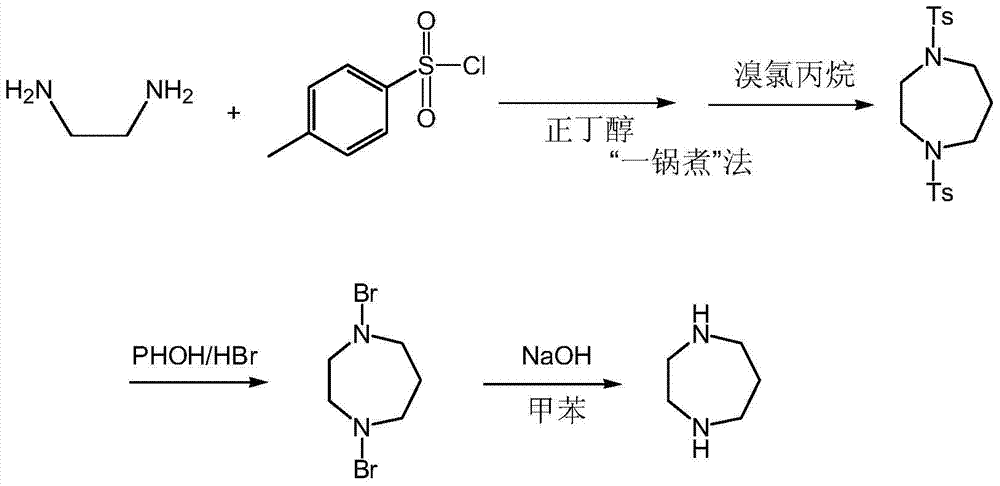
[0031] The method for preparing homopiperazine is as follows:
[0032] (1) The first step: take ethylenediamine and n-butanol and p-toluenesulfonyl chloride to react, then add 50% sodium hydroxide, stir, heat to reflux, then add bromochloropropane and 50% sodium hydroxide solution, and react Centrifuge for 1-3 hours to obtain N,N'-di-p-toluenesulfonyl homopiperazine;
[0033] (2) The second step: sequentially put phenol, hydrobromic acid and N,N'-di-p-toluenesulfonyl homopiperazine into the reaction tank for reaction, concentrate under reduced pressure, remove the remaining phenol and hydrobromic acid, add ethanol , centrifuged to obtain homopiperazine hydrobromide;
[0034] (3) The third step: drop homopiperazine hydrobromide and 50% sodium hydroxide solution into the reaction tank, stir at room temperature, add toluene in the reaction tank, reflux, centrifuge, collect the filtrate, distill under reduced pressure, collect 90 ℃-110℃ or so distillate to obtain homopiperazine....
Embodiment 2
[0036] The method for preparing homopiperazine is as follows:
[0037](1) The first step: put 20-30kg ethylenediamine and 60-80kg n-butanol into the reaction tank, add 140-180kg p-toluenesulfonyl chloride and 70-90kg 50% sodium hydroxide at 25°C, and stir 10-20 minutes, heat to reflux, then add 60-70kg bromochloropropane and 70-90kg 50% sodium hydroxide solution, the pH of the reaction system is 10-12, heat to reflux, react for 1.5-2.5 hours, centrifuge, and dry Get N,N'-di-p-toluenesulfonyl homopiperazine;
[0038] (2) The second step: successively put 60-80kg phenol, 90-110kg hydrobromic acid (content: 45-60%) and 130-150kg N, N'-di-p-toluenesulfonyl homopiperazine into the reaction tank, and reflux React for 4-6 hours, 120°C, concentrate under reduced pressure at 5 mgHg, remove the remaining phenol and hydrobromic acid, then add 40-60 kg of ethanol, stir and crystallize overnight, centrifuge, and dry to obtain homopiperazine hydrobromide;
[0039] (3) The third step: put ...
Embodiment 3
[0041] The method for preparing homopiperazine is as follows:
[0042] (1) The first step: Get 25kg of ethylenediamine and 75kg of n-butanol and put it into a 500L enamel reaction tank. At 25°C, add 160kg of p-toluenesulfonyl chloride and 80kg of 50% sodium hydroxide, stir for 15 minutes, and heat to reflux , then add 65kg bromochloropropane and 80kg 50% sodium hydroxide solution, the pH of the reaction system is 12, heat to reflux, react for 2 hours, centrifuge, and dry to obtain 143kg of N,N'-di-p-toluenesulfonyl homopiperazine , yield 91.1%, content 99.1%;
[0043] (2) The second step: put 70kg of phenol, 100kg of hydrobromic acid (content: 60%) and 140kg of N,N'-di-p-toluenesulfonyl homopiperazine into a 500L enamel reaction tank in sequence, and reflux for 5h at 120°C , concentrated under reduced pressure at 5 mgHg, removed remaining phenol and hydrobromic acid, then added 50kg of ethanol, stirred and crystallized overnight, centrifuged, dried to obtain 78.7kg homopipera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com