Hemicyanine compound for detecting polarity of mitochondria
A technology of cyanine compounds and mitochondria, applied in the field of fluorescent probe compounds, can solve the problems of no literature reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The synthesis of embodiment 1. fluorescent probe compound BOB:
[0055] It is the synthetic method of representative compound BOB of the present invention, and its synthetic route is as follows:
[0056]
[0057] The synthetic method comprises the following steps
[0058] (1) 0.85g benzyl bromide and 0.90g 2-methylbenzothiazole were reacted at 65°C for 3h under nitrogen protection, and cooled to room temperature. After the solid was completely settled, it was vacuum filtered, the obtained solid was washed with ether, and finally the solid was dried in vacuum to obtain the white intermediate 1a;
[0059] In this step, no solvent is needed when 2-methylbenzothiazole reacts with benzyl bromide;
[0060] This step reacts under inert gas protection;
[0061] (2) 0.97g 4-diethylamino salicylaldehyde, 1.6g diethyl malonate and 1.0mL piperidine were stirred and refluxed in 30mL ethanol solution for 6 hours (80°C). After ethanol was distilled off under reduced pressure, 2...
Embodiment 2
[0067] Embodiment 2. Synthesis of fluorescent probe compound BOB-1:
[0068]
[0069] Dissolve 0.67g of intermediate 1a in ethanol, add 0.23g of intermediate 2b, and heat to reflux to form a purple dye BOB-1. After the reaction was completed, the solvent was distilled off. The product is preferably purified by chromatographic column separation using dichloromethane / methanol as the eluent. The product was characterized by NMR and high-resolution mass spectrometry. R 1 for H, R 2 for para-CH 3 : 1 H NMR (400MHz, MeOD): 8.29(s, 2H), 8.24(d, J=7.3Hz, 1H), 8.16(d, J=8.3Hz, 1H), 8.08(d, J=15.0Hz, 1H) ,7.75(t,J=7.2Hz,1H),7.53(s,1H),7.38(d,J=10.8Hz,5H),6.88(dd,J=9.1,2.4Hz,1H),6.62(d, J=2.3Hz,1H),6.03(s,2H),3.58(q,J=7.1Hz,4H),2.34,(s,3H),1.26(dd,J=12.5,5.5Hz,10H).
[0070] 13 C NMR (101MHz, MeOD): δ195.60, 137.40, 123.98, 122.67, 121.29, 116.29, 93.76, 82.22, 80.91, 28.35, 26.27, 21.47, 19.50.
[0071] HRMS-ESI: m / z calcd M + for C 30 h 29 N 2 o 2 S + , 481.1944; fou...
Embodiment 3
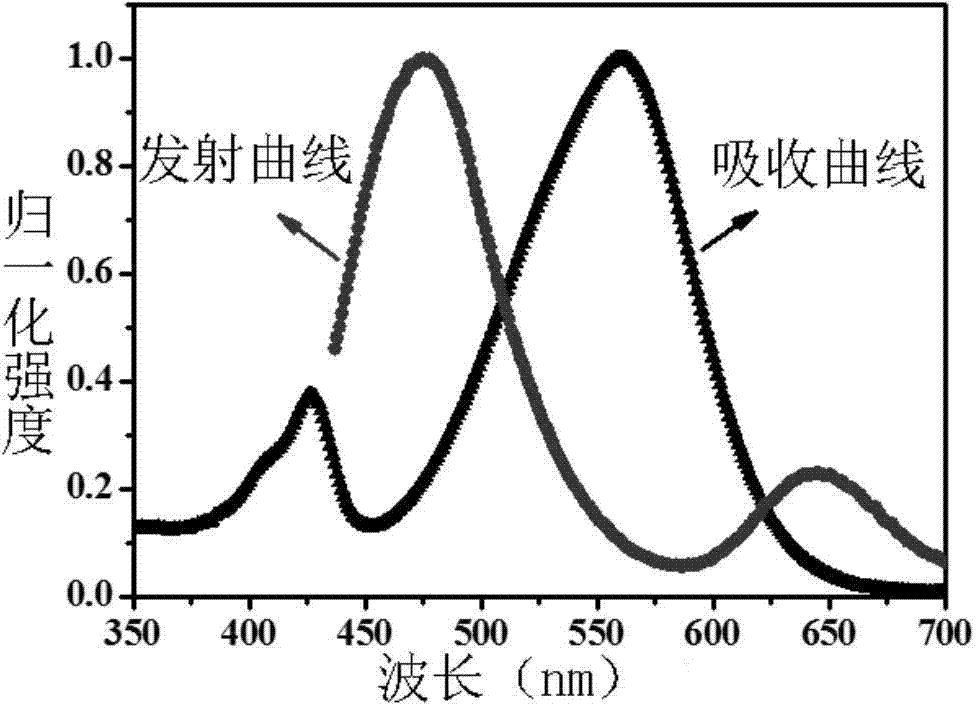
[0072] Example 3. Absorption and emission spectra of dye BOB in ethanol
[0073] The dye BOB was dissolved in ethanol, respectively, to prepare solutions with a final concentration of 5.0 μM.
[0074] The test results are attached figure 1 As shown, the maximum absorption of the dye BOB in ethanol is at 425 / 560nm, and the maximum emission spectrum is at 467 / 645nm.
[0075] The instruments used are ultraviolet-visible spectrophotometer, model: Perkin Elmer Lambda 35UV / VIS; fluorescence spectrophotometer, model: FL0812M018.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com