Method for efficiently producing L-glutamate oxidase
A glutamate oxidase, high-efficiency expression technology, applied in the fields of fermentation engineering and enzyme engineering, can solve the problems of α-KG difficulty, safety problems, raw material sources, etc., and achieve the effect of high-efficiency expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Optimization of Lactose Inducing Conditions
[0030] Using the recombinant strain FMME089 (Panqing Niu, Enzymatic production of α-ketoglutaric acid from L-glutamic acid via L-glutamateoxidase, Journal of Biotechnology, 2014, 56-62) constructed in our laboratory as the starting strain, the conditions for lactose induction were optimized.
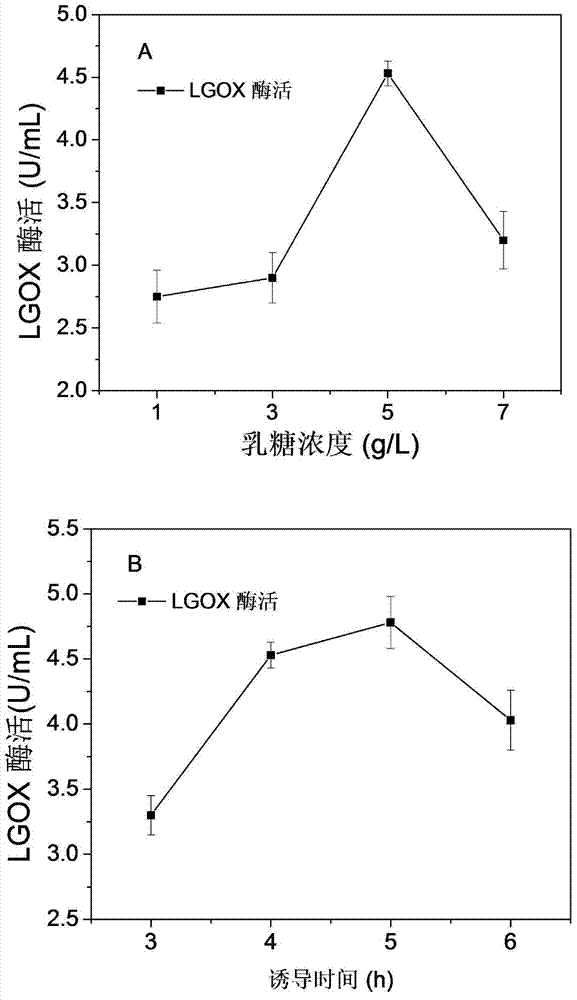
[0031] 1) Inoculate the recombinant strain in LB medium, OD 600 When it is between 0.5-0.6, add 1, 3, 5, 7 g / L lactose respectively to induce 4 hours at 37°C, and compare the expression of LGOX. It was found that the lactose induction effect of 5g / L was the best and the enzyme activity reached 4.53U / mL ( figure 1 A).
[0032] 2) On the basis of the optimal lactose induction concentration, the expression levels of LGOX were measured at 3, 4, 5, and 6 hours after induction, respectively. The results showed that the effect of induction for 5h was the best, and the enzyme activity reached 4.73U / mL ( figure 1 B).
Embodiment 2
[0033] Embodiment 2 batch fermentation
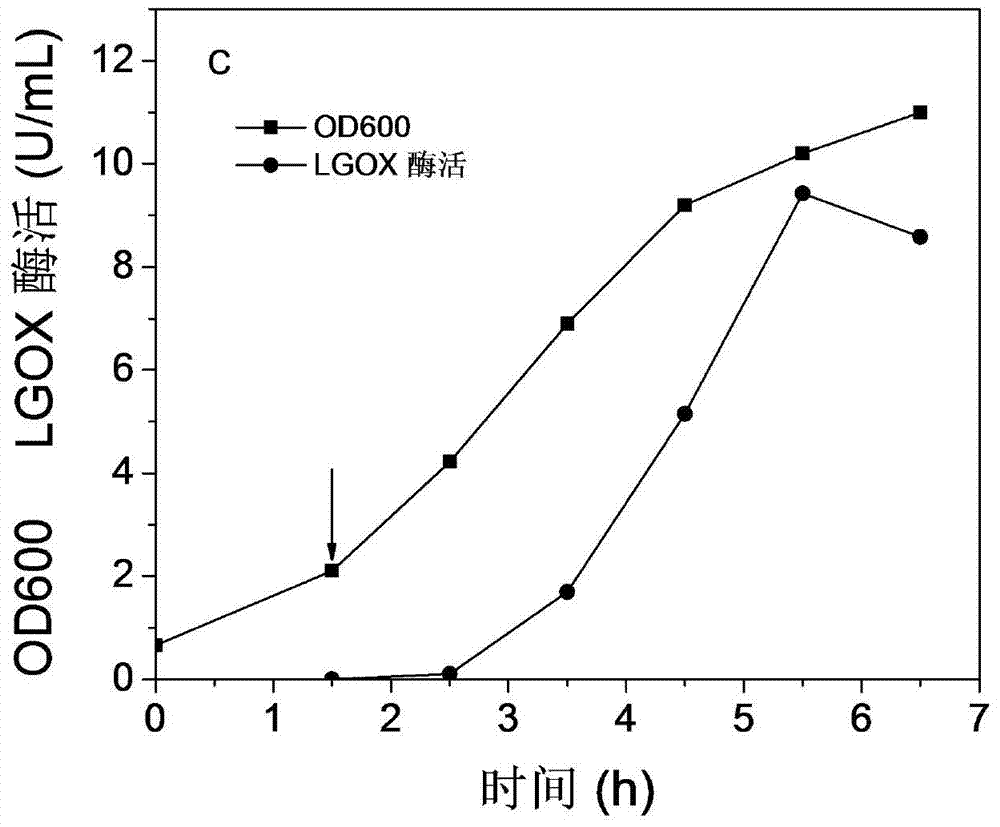
[0034] The recombinant bacteria were first inoculated on the LB seed medium for 10-12 hours, and then transferred to a 5L fermenter containing 2LLB medium with a 4% inoculation amount. The initial fermentation conditions are: rotation speed 300rpm, temperature 37°C, ventilation rate 1vvm. When OD600=0.5-0.7, the final concentration of 5g / L lactose was used for induction, and the bacterial concentration and enzyme activity expression at different time points were measured. The results showed that the enzyme activity reached the maximum value of 9.43U / mL 4 hours after induction.
Embodiment 3
[0035] Embodiment 3 fed-batch fermentation based on DO-stat
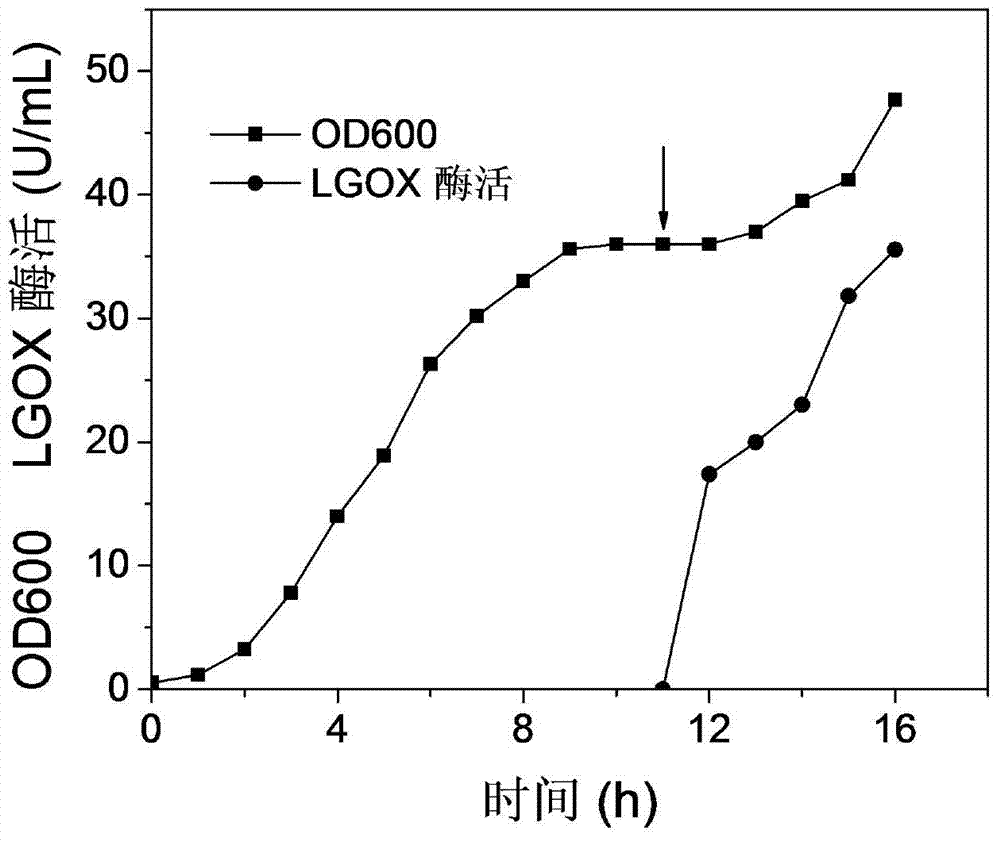
[0036] The starting condition of the fermenter is the same as that of batch fermentation. Before feeding, control the DO to be above 30%. When the DO suddenly rises, start feeding, and control the DO-related feeding. Whenever the DO is higher than 30%, it will be fed for a while. material. The composition of feed solution was 500g / L glycerol, and 5g / L lactose was used for induction when the cell concentration no longer increased. After 16 hours of fermentation, the cell concentration OD=47.7, and the enzyme activity was 35.55U / mL ( image 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com