Attenuated streptococcus suis vaccines and methods of making and use thereof
A technology of Streptococcus suis and strains, applied in the direction of vaccines, veterinary vaccines, bacteria, etc., can solve the problem of lack of effective vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] Example 1 - Generation and genome analysis of attenuated S. suis
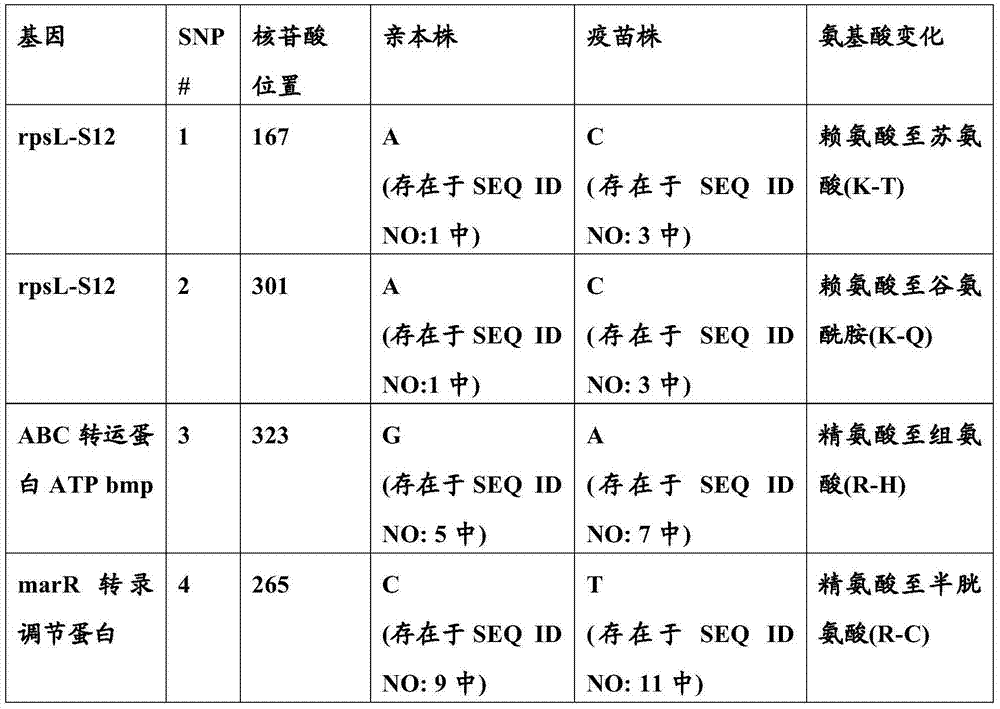
[0111] To develop a vaccine with broad protection against S. suis infection, the US field strain (parental strain, Newport Laboratory No. 8-1433-1, SEQ ID NO: 9) was isolated from pig brain cotton swabs. Based on biochemical reactions and agglutination tests, the isolate was identified as S. suis serotype 2. Attenuation of this parental strain was accomplished by chemical mutagenesis using acriflavine hydrochloride as described by Sinha et al. Briefly, S. suis parental cells were incubated for 18 hours in tryptic casein soybean broth supplemented with 10 [mu]M / ml acriflavine hydrochloride containing 5% sheep blood. Surviving cells were reisolated on sheep blood agar plates. 20 single colonies were selected and grown separately in the same liquid medium. One of these 20 cultures was selected and injected (1.76 × 10 9 cells / ml) into pigs to determine virulence. Ten pigs were inoculated orally and obse...
Embodiment 2
[0120] Example 2 - Efficacy of attenuated S. suis vaccine in pigs
[0121] General protocol for efficacy studies: Colony forming units (CFU) / ml were determined for each vaccine dilution throughout the titration period before and after vaccination.
[0122] All pigs enrolled in these studies were obtained from Midwest Research Swine, Gibbon, MN and were healthy and normal upon arrival. The herd was considered to be a highly healthy source herd with no history of S. suis infection and negative for porcine reproductive and respiratory syndrome virus (PRRSV). The source herd piglets had a history of susceptibility to S. suis challenge in previous studies. Pigs are received at 17-24 days of age. Animals were observed prior to inoculation and were considered clinically normal because they lacked signs of disease, including coughing, abdominal respiration, and / or lameness. Individual pigs were used as experimental units in these studies and ear-tagged prior to arrival. Pigs were ra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com