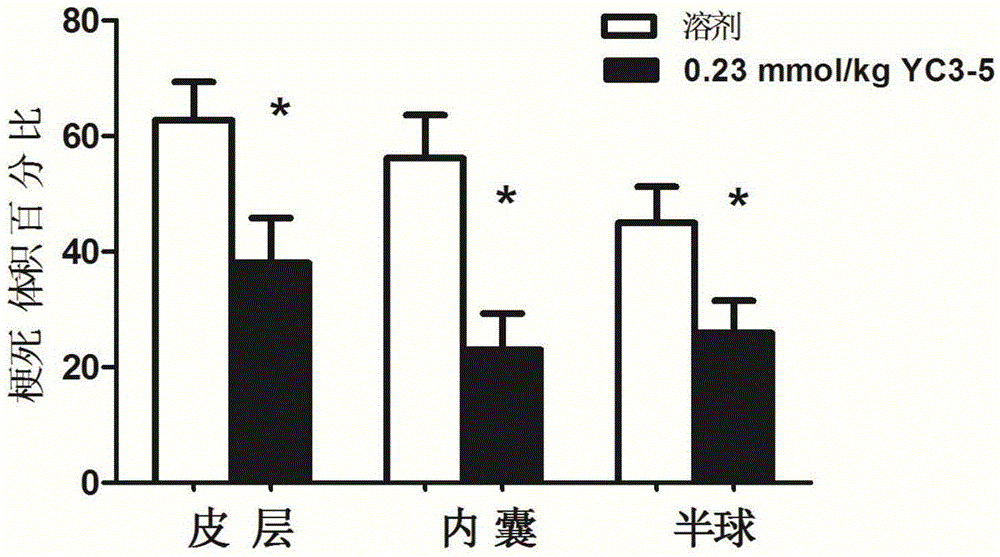
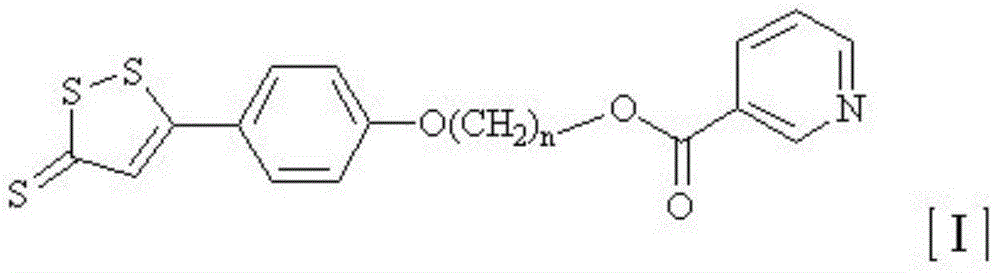
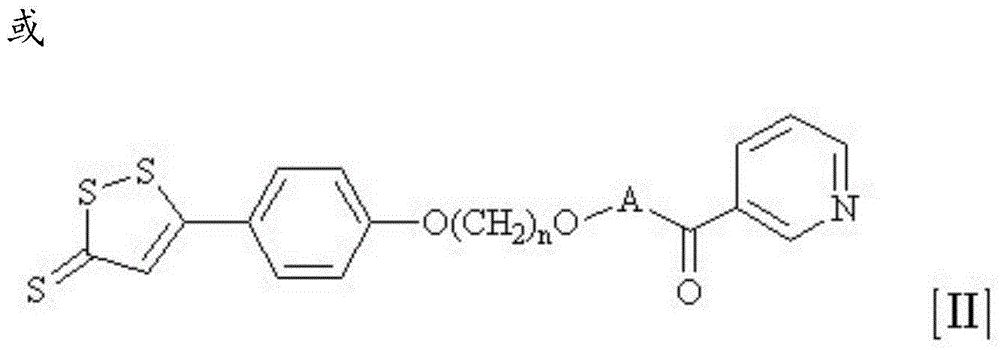
Nicotinic acid derivative and application thereof
A derivative, the technology of nicotinic acid, applied in the field of prevention or treatment of stroke diseases, can solve problems such as difficult to achieve the expected effect, and achieve good preventive and/or therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] [Example 1] Synthesis of Nicotinic Acid 2-[4-(3H-1,2-dithiol-3-thiol-5-yl)phenoxy]ethyl ester (YC3-1)
[0055]
[0056] ADT-OH (1.000g, 8.13mmol), 2-bromoethyl nicotinate (0.757mL, 8.13mmol) and K2CO3 (2.240g, 16.26mmol) were cast in 60mL of acetone, and stirred at reflux for 18h. After the reaction was completed, filter, evaporate the solvent to dryness, and perform column chromatography [petroleum ether (60-90):ethyl acetate=10:1 (v / v)] to obtain a red solid with a yield of 75.9%.
[0057] 1 H NMR (400MHz, CDCl 3 ), δ (ppm): 9.25 (d, J = 1.5Hz, 1H, ArH), 8.80 (dd, J = 4.8, 1.7Hz, 1H, ArH), 8.32 (d, J = 8.0Hz, 1H, ArH) ,7.63(d,J=8.8Hz,2H,ArH),7.41(dd,J=8.0,5.0Hz,1H,ArH),7.39(s,1H,CH,ArH),7.03(d,J=8.9Hz ,2H,ArH),4.77–4.73(m,2H,CH 2 ),4.42–4.38(m,2H,CH 2 ).
[0058] 13 C NMR (400MHz, CDCl 3 ), δ (ppm): 215.32, 172.81, 165.27, 161.79, 153.87, 151.16, 137.31, 134.98, 128.80, 125.75, 124.90, 123.48, 115.69, 66.25, 63.40.
[0059] MS: Calcd. For C 17 h 13 NO 3...
Embodiment 2
[0060] [Example 2] Synthesis of Nicotinic Acid 3-[4-(3H-1,2-dithiol-3-thiol-5-yl)phenoxy]propyl ester (YC3-2)
[0061]
[0062] Referring to the method of Example 1, it was prepared from 3-bromopropyl nicotinate and ADT-OH, and the product was a red solid with a yield of 70.4%.
[0063] 1 H NMR (300MHz, CDCl 3 ), δ (ppm): 9.23 (s, 1H), 8.79 (d, J = 3.1Hz, 1H, ArH), 8.30 (d, J = 7.9Hz, 1H, ArH), 7.61 (d, J = 8.6Hz ,2H,ArH),7.42(dd,J=4.3,3.7Hz,1H,ArH),7.39(s,1H,ArH),6.98(d,J=8.6Hz,2H,ArH),4.59(t,J =6.1Hz,2H,CH 2 ), 4.21(t, J=5.8Hz, 2H, CH 2 ),2.36–2.30(m,2H,CH 2 ).
[0064] 13 C NMR (400MHz, CDCl 3 ), δ (ppm): 215.02, 172.94, 165.17, 161.98, 153.56, 150.83, 137.07, 134.63, 128.61, 125.93, 124.31, 123.37, 115.38, 64.76, 62.06, 28.51.
[0065] MS: Calcd. For C 18 h 15 NO 3 S 3 [M+H] + 389.0214,Found: 389.0293.
Embodiment 3
[0066] [Example 3] Synthesis of nicotinic acid 4-[4-(3H-1,2-dithiol-3-thiol-5-yl)phenoxy]butyl ester (YC3-3)
[0067]
[0068] Referring to the method of Example 1, it was prepared from 4-bromobutyl nicotinate and ADT-OH, and the product was a red solid with a yield of 70.4%.
[0069] 1 H NMR (300MHz, CDCl 3 ),δ(ppm):9.22(s,1H,ArH),8.78(s,1H,ArH),8.30(d,J=5.9Hz,1H,ArH),7.60(d,J=8.6Hz,2H, ArH), 7.38(m, 2H, ArH, CH), 6.97(d, J=7.9Hz, 2H, ArH), 4.46(s, 2H, CH 2 ),4.11(s,2H,CH 2 ),2.01(s,4H,CH 2 ).
[0070] 13 C NMR (400MHz, CDCl 3 ), δ (ppm): 215.11, 173.09, 165.30, 162.29, 153.52, 150.90, 137.11, 134.66, 128.67, 126.17, 124.22, 123.41, 115.48, 67.75, 65.01, 25.84, 25.53.
[0071] MS: Calcd. For C 19 h 17 NO 3 S 3 [M+H] + 404.0, Found: 403.9.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com