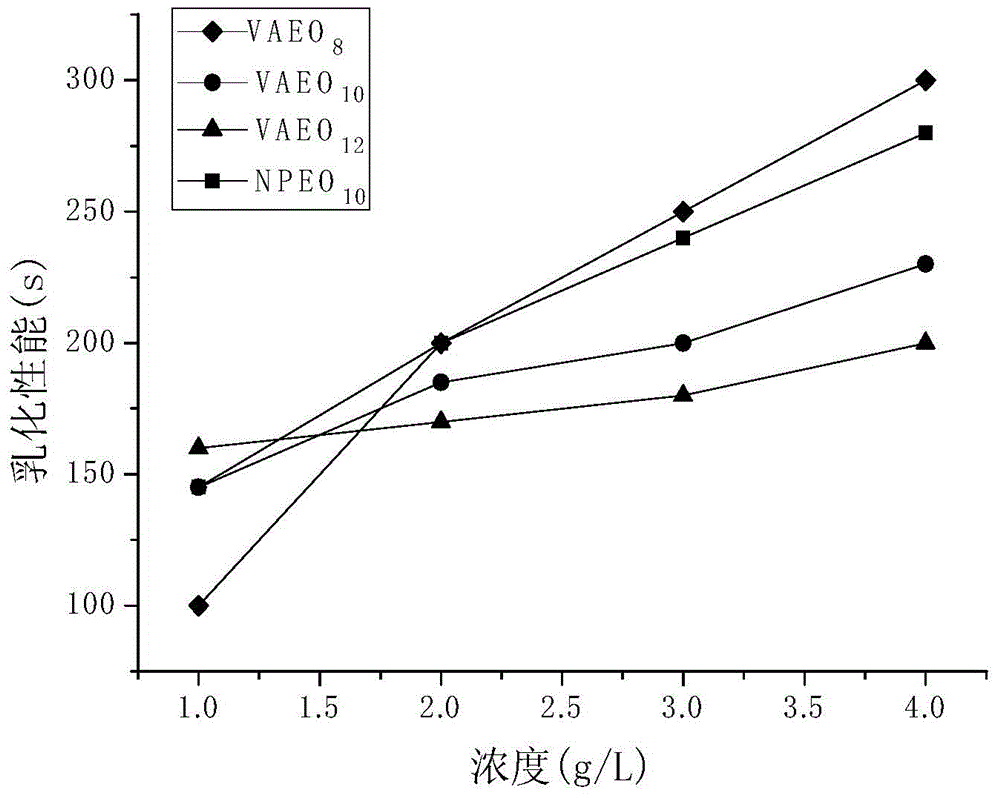
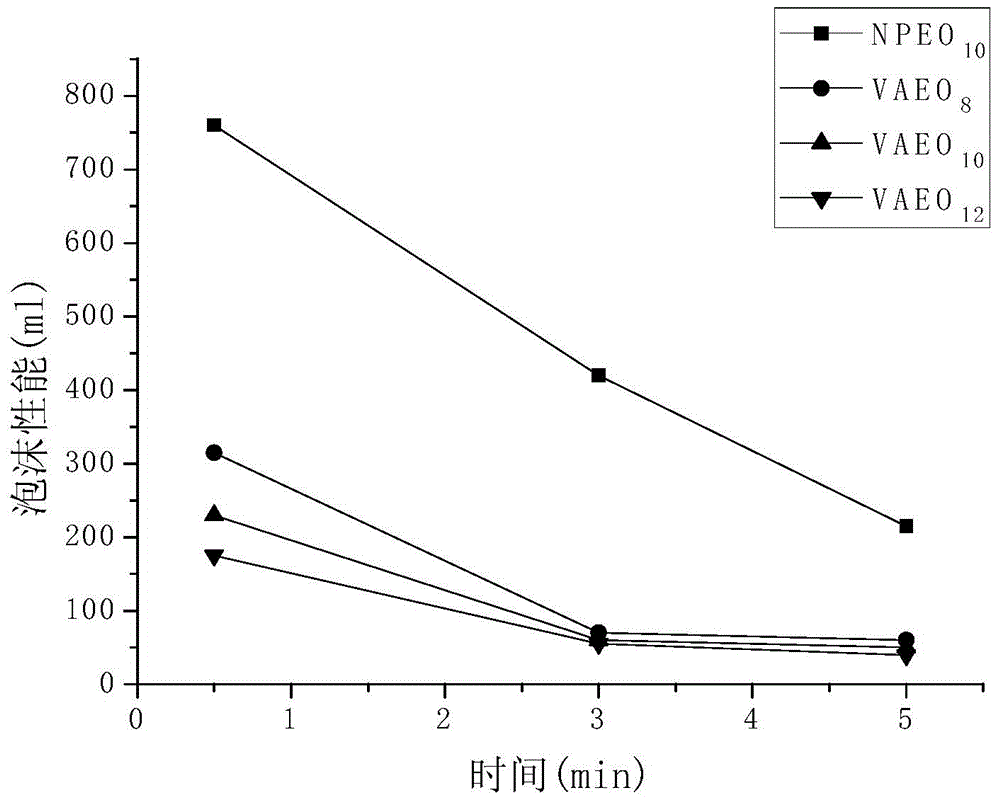
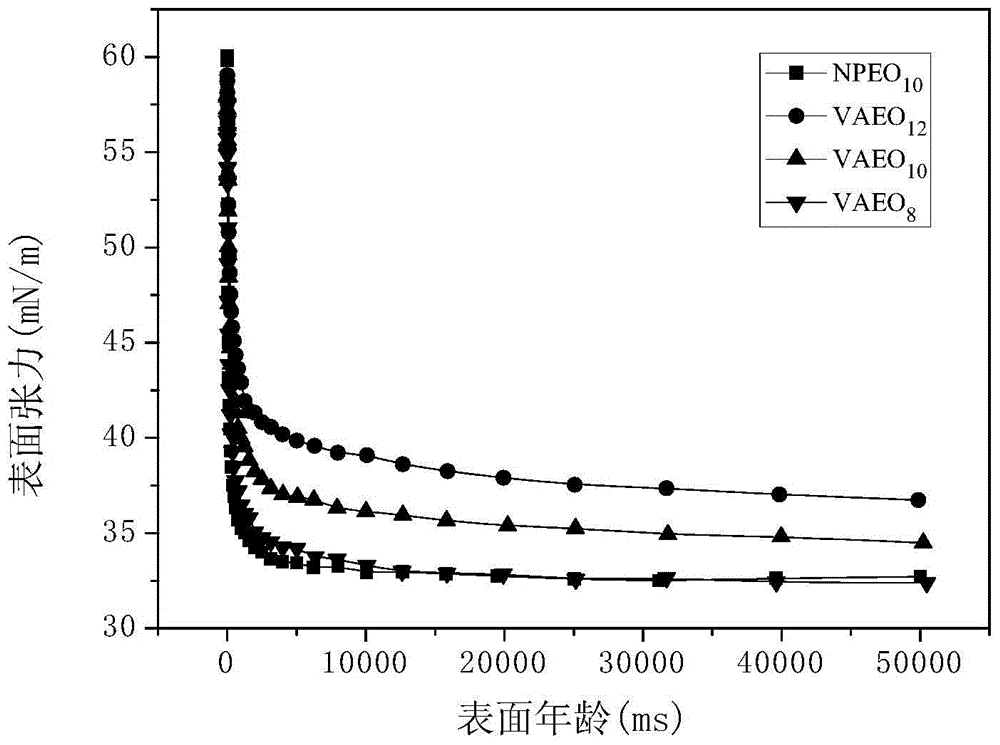
Dissoluble vanillin based non-ionic surfactant, as well as preparation method and application of dissoluble vanillin based non-ionic surfactant
A surfactant, vanillin-based non-ionic technology, applied in the field of decomposable vanillin-based non-ionic surfactant and its preparation and application, can solve aquatic organisms and human health threats, poor biodegradability, environmental burden, etc. problem, to achieve the effect of simple and feasible purification process, reduced harm and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] (1) Synthesis of vanillin n-butanol acetal
[0046] A three-necked flask was charged with 0.1 mol of vanillin, 0.3 mol of n-butanol, 0.5 g of 85% phosphoric acid, and 40 ml of n-hexane. Install the thermometer, water separator and tee joint. Add 10ml of n-hexane into the water trap to the branch pipe of the water trap, and install a serpentine reflux condenser. Vacuum with a circulating water pump and blow in nitrogen for replacement, repeat three times. The oil bath is heated to reflux, and the reaction is until no more water is formed. Cool to room temperature, add saturated potassium bicarbonate solution to neutralize, separate liquids to obtain an organic phase, add anhydrous sodium sulfate overnight, and rotatory steam to obtain a yellow viscous crude product. Add saturated sodium bisulfite solution for further purification to obtain the product. The acetal content in the crude product was 79%. After purification with saturated sodium bisulfite solution, the aceta...
Embodiment 2
[0050] (1) Vanillin 1,3-octanediol acetal
[0051] A three-necked flask was charged with 0.1 mol vanillin, 0.15 mol 1,3 octanediol, 0.5 g 85% phosphoric acid, and 40 ml n-hexane. Install the thermometer, water separator and tee joint. Add 10ml n-hexane to the water trap to the branch pipe of the water trap, and install a serpentine reflux condenser. Vacuum with a circulating water pump and blow in nitrogen for replacement, repeat three times. The oil bath is heated to reflux, and the reaction is until no more water is formed. Cool to room temperature, add saturated potassium bicarbonate solution to neutralize, separate liquids to obtain an organic phase, add anhydrous sodium sulfate overnight, and rotate to steam to obtain a yellow viscous crude product. The product is further purified by adding saturated sodium bisulfite solution. The acetal content in the crude product is 80%. After the saturated sodium bisulfite solution is purified, the acetal purity is determined to be 9...
Embodiment 3
[0055] (1) Vanillin 1,2-octanediol acetal
[0056] A three-necked flask was charged with 0.1 mol vanillin, 0.15 mol 1,2 octanediol, 0.5 g of 85% phosphoric acid, and 40 ml of n-hexane. Install the thermometer, water separator and tee joint. Add 10ml of n-hexane to the water trap to the branch pipe of the water trap, and install a serpentine reflux condenser. Vacuum with a circulating water pump and blow in nitrogen for replacement, repeat three times. The oil bath is heated to reflux, and the reaction is until no more water is formed. Cool to room temperature, add saturated potassium bicarbonate solution to neutralize, separate liquids to obtain an organic phase, add anhydrous sodium sulfate overnight, and rotate to steam to obtain a yellow viscous crude product. The product is further purified by adding saturated sodium bisulfite solution. The acetal content in the crude product was 83.4%. After purification with saturated sodium bisulfite solution, the acetal purity determi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com