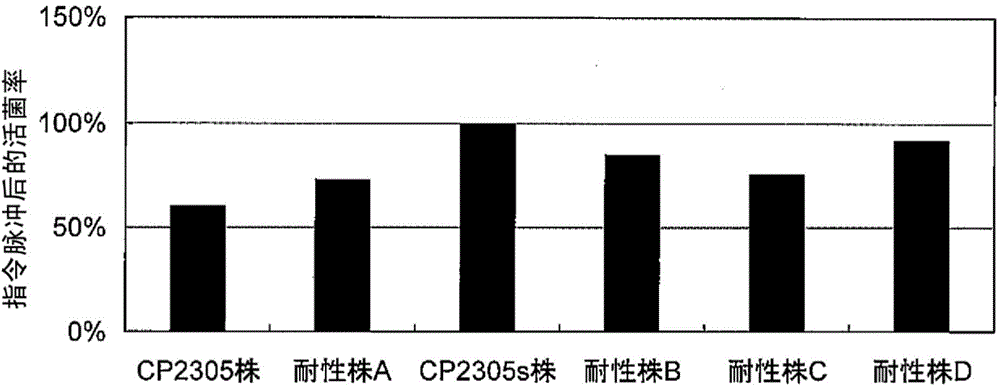
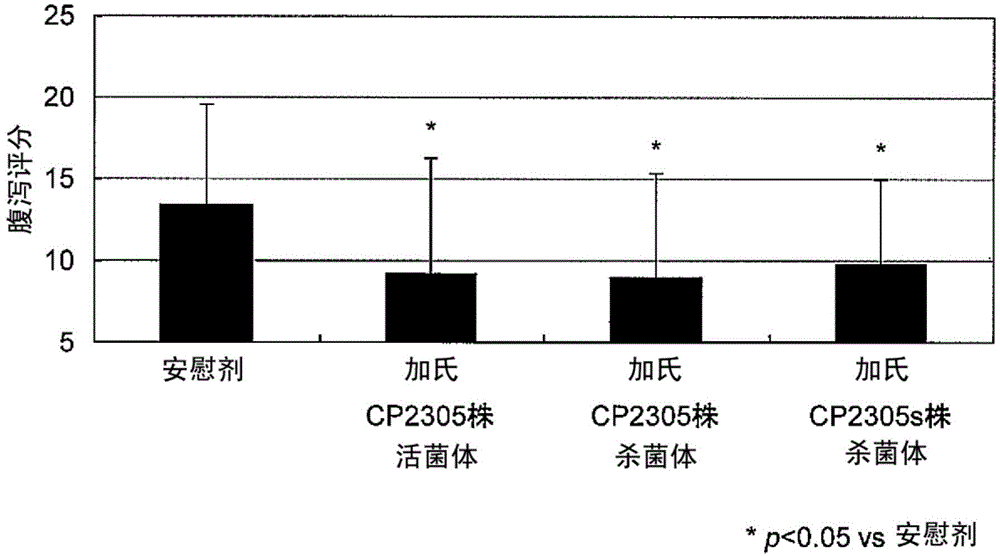
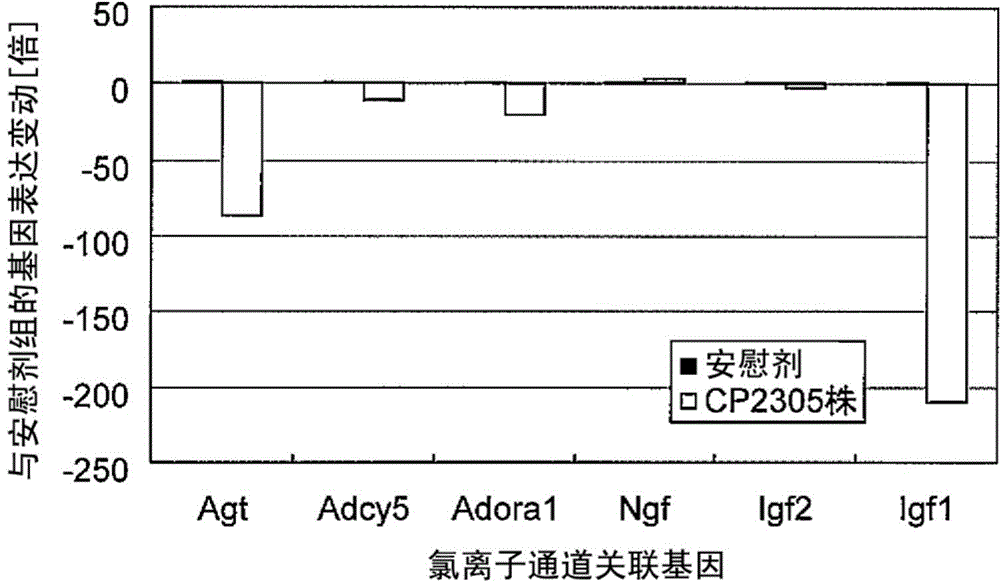
Stress-induced bowel disorder-relieving agent comprising specific lactobacillus gasseri strain or treatment product thereof
A technology of Lactobacillus gasseri, stress, used in the field of mitigants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0142]
[0143] Inoculate Lactobacillus gasseri CP2305 strain (number of bacteria 10 3 a ~ 10 4 ), cultured at 37°C for 19 hours.
[0144] After culturing for 19 hours, this 15-ml polypropylene test tube with an inner diameter of 15 mm was inserted into a sufficient amount of ice above the liquid level in the test tube, rapidly cooled (on ice), and kept for 5 hours. This cultivation and cooling were repeated 80 times, and a strain with high low temperature tolerance was bred.
[0145] In this specification, "on ice" refers to a state in which 10 ml of culture solution is placed in a 15 ml test tube made of polypropylene with an inner diameter of 15 mm, and the test tube is cooled by inserting a sufficient amount of ice above the liquid level in the test tube. .
[0146] In addition, the composition of the "food additive medium" used in this specification is as follows (Table 1).
[0147] [Table 1]
[0148] Composition of food additive medium
[0149] compositi...
Embodiment 2
[0157]
[0158] It should be noted that, as a method for producing a low-temperature tolerant strain, for example, the CP2305 strain tends to have a reduced survival rate at temperatures below 10°C. Therefore, as a method for producing a low-temperature tolerant strain, the target strain is cultured in a culture solution (for example: a food additive medium) at a moderate culture temperature (for example: about 37°C) and for a moderate culture time (for example: about 19 hours). After culturing, the culture solution is exposed for a moderate time at a temperature below 10°C, preferably below 7°C, more preferably below 5°C, more preferably below 3°C, below 2°C, or below 1°C (for example: 5 hours), and, if necessary, by repeating their cultivation and exposure to low temperature, strains resistant to low temperatures at the target temperature were produced.
Embodiment 3
[0160] SD male rats (4-week-old, manufactured by Charles River Laboratories) were bred under a 12-hour light-dark cycle (lighting on for 12 hours from 8 o'clock) for 1 week, and then allowed to freely ingest the test bacterial cell mixture bait for 3 weeks as follows.
[0161] a) CRF-1 powder bait from Oriental Yeast Co., Ltd. was used as bait.
[0162] b) The number of bacteria contained in 25 g of the above-mentioned bait (CRF-1) is 2.0×10 10 The freeze-dried powder (0.25 g of the freeze-dried powder was compounded with respect to the bait CRF-1: 25 g) of each test cell was used as the test cell compounded bait.
[0163] c) Allow them to freely ingest the mixed bait of the test bacteria for 3 weeks.
[0164] d) The food intake of the bacteria in this test is estimated to be about 2.0×10 10 pcs / day.
[0165] e) Let 19 animals in each group ingest.
[0166] After 3 weeks, a CRF (Corticotropin Releasing Factor, manufactured by Anaspec, USA) solution was intraperitoneally ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com