Application of Matrine in preparation of medicine for treating cerebral arterial thrombosis
A technology for ischemic stroke and matrine, which is applied in the application field of matrine for the treatment of ischemic stroke, can solve the problems of lag in clinical treatment drug research, difficult to achieve treatment methods, limited treatment options, etc. The effect of reducing the volume of cerebral infarction, reducing the rate of apoptosis, and improving neurological dysfunction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Matrine is used as a medicine for treating ischemic stroke, and the structural formula of matrine is as shown in formula (1):
[0042]
[0043] Among them, ischemic stroke is ischemic stroke in the acute stage, and the single application dose of matrine is limited to the dose that does not cause blood sugar drop. The single application dose of matrine is 7.5mg / kg body weight of mice, and the The dosage form is injection dosage form.
Embodiment 2
[0045] Matrine is used as a medicine for treating ischemic stroke, and the structural formula of matrine is as shown in formula (1):
[0046]
[0047] Among them, ischemic stroke is ischemic stroke in the repair period, and the single application dose of matrine is limited to the dose that does not cause blood sugar drop. The single application dose of matrine is 15 mg / kg body weight of mice, and the dosage form of the drug is It is in powder form for injection.
Embodiment 3
[0049] Matrine is used as a medicine for treating ischemic stroke, and the structural formula of matrine is as shown in formula (1):
[0050]
[0051] Among them, ischemic stroke is ischemic stroke in the repair period, and the single application dose of matrine is limited to the dose that does not cause blood sugar drop. The single application dose of matrine is 30 mg / kg body weight of mice, and the dosage form of the drug is It is in powder form for injection.
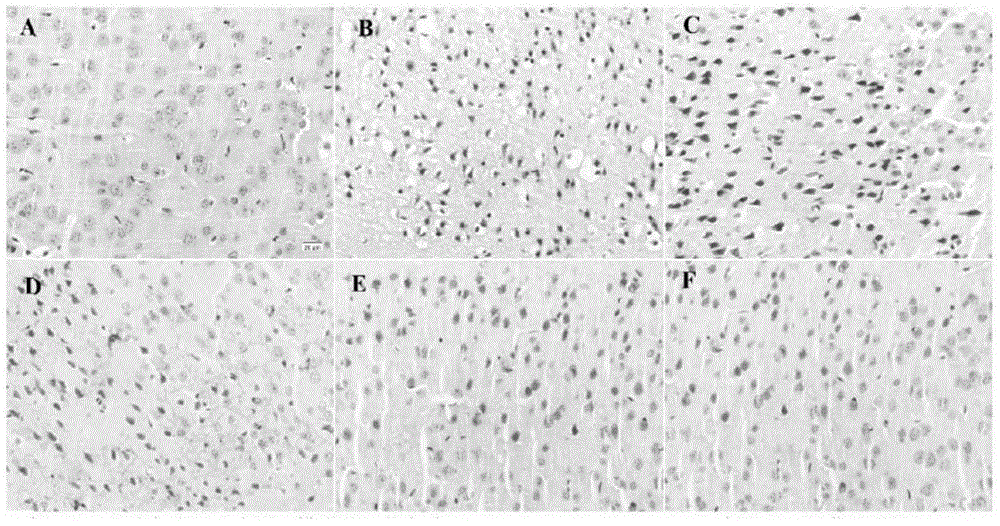
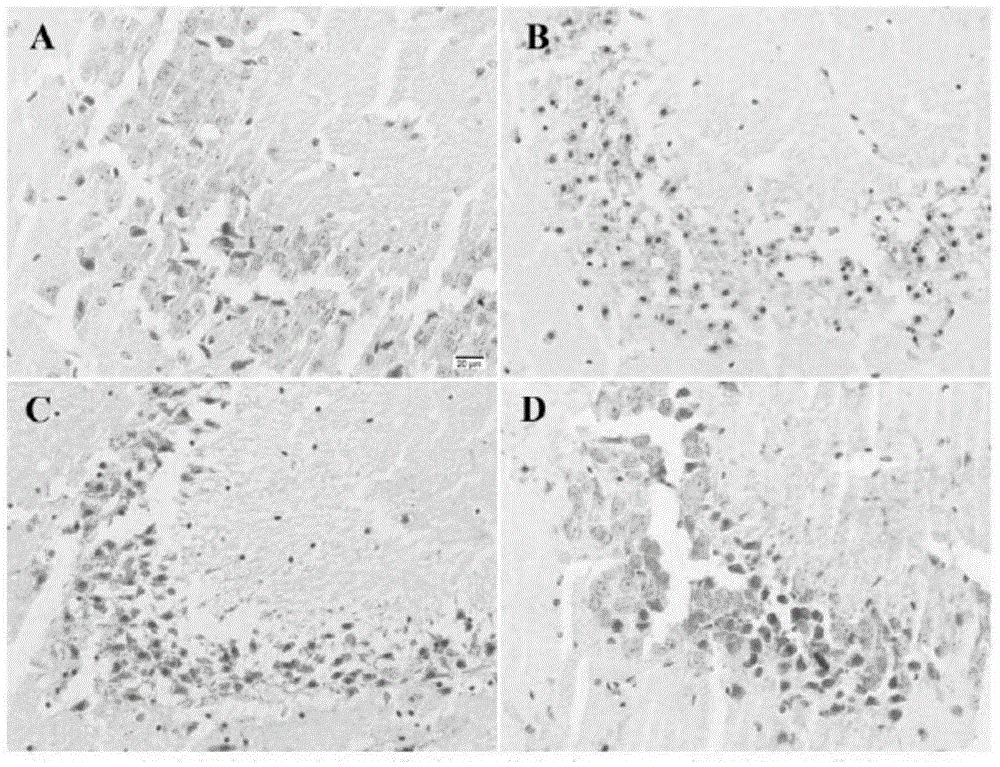
[0052] The following animal experiment has further illustrated the effect of above-mentioned embodiment 1-3:
[0053] 1. Experimental materials
[0054] 1.1 Animal handling
[0055]Male ICR mice aged 2-3 months, weighing 25-30 g, were purchased from the Experimental Animal Center of Ningxia Medical University, animal production license number: NCXK (Ning) 2013-0005. Feeding conditions include standard feed, tap water, room temperature maintained at (24±2)°C, humidity 50-60%, and daily light and dark times of 12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com