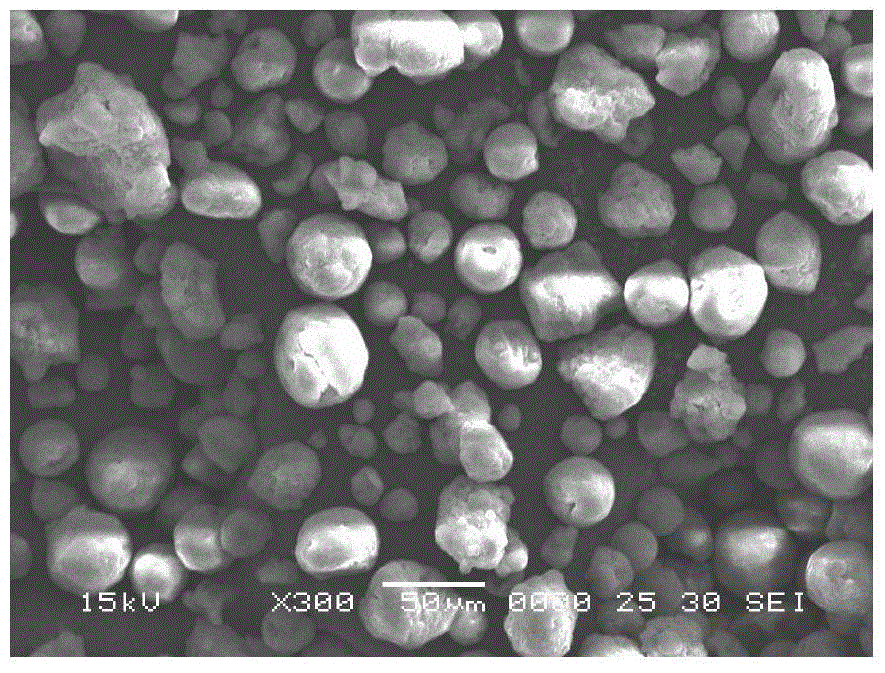
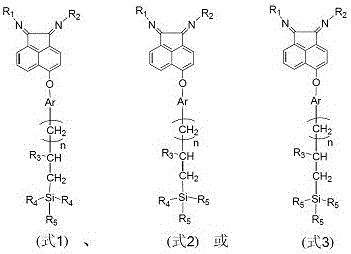
Supported α-diimine compound and its application in olefin polymerization
A diimine compound, supported technology, applied in nickel organic compounds, silicon organic compounds, compounds containing elements of Group 8/9/10/18 of the periodic table, etc., can solve the problem of poor thermal stability and difficult polymer morphology. Control, the amount of co-catalysts and other issues, to achieve good thermal stability, strong chemical bond force, reduce the effect of the impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Synthesis and preparation of 5-{4-[3-(silica oxydimethylsilyl)propoxy]phenoxy}acenaphthoquinonebis(2,6-diisopropyl)benzimine support e1 The route is as follows:
[0036]
[0037] The specific operation steps are as follows:
[0038] Synthetic references of 5-bromoacenaphthylquinone [J Am Chem Soc,2013,135(46):17469], synthetic references of 4-allyloxyphenol a1 [J Med Chem,2011,54(13): 4659]
[0039] Synthesis of 5-(4-allyloxyphenoxy)acenaphthenequinone b1:
[0040] Add 12.7g (48.6mmol) of 5-bromoacenaphthylquinone in the reaction flask of 100ml, 13.8g (100mmol) K 2 CO 3 , 33ml of dry DMF, and start stirring. Add 15g (100mmol) a1 during the stirring process, and react at 60°C. The reaction is stopped after the chromatographic trace traces that the reactants have completely reacted. Pour the dark brown solution of the reaction into a saturated NaCl solution to become a brown suspension, extract 2-3 times with dichloromethane, and use anhydrous MgSO for the organi...
Embodiment 2
[0050] Synthesis and preparation of 5-{4-[3-(silicadioxymethylsilyl)propoxy]phenoxy}acenaphthoquinone bis(2,6-diisopropyl)phenylimide g1 The route is as follows:
[0051]
[0052] Preparation process of 5-{4-[3-(dichloromonomethylsilyl)propoxy]phenoxy}acenaphthoquinone bis(2,6-diisopropyl)phenylimine f1 and compound d1 in Example 1 Same, where dichloromonomethylsilane is used instead of monochlorodimethylsilane; SiO 2 / MgCl 2 The preparation process of the composite carrier is the same as in Example 1, wherein MgCl 2 and SiO 2 The masses are all 0.5 g; the specific synthesis steps of the load g1 are the same as the synthesis steps of the load e1 in Example 1. Elemental analysis of load g1: C, 22.12%; N, 1.10%.
Embodiment 3
[0054] Synthesis of 5-{4-[3-(Silicatrioxysilyl)propoxy]phenoxy}acenaphthoquinone bis(2,6-diisopropyl)phenylimide i1
[0055] Its preparation route is as follows:
[0056]
[0057] 5-{4-[3-(trichlorosilicon) propoxyl]phenoxy}acenaphthenequinone bis(2,6-diisopropyl)phenylimine h1 is prepared in the same way as compound d1 in Example 1, wherein Using trichlorosilane instead of monochlorodimethylsilane; SiO 2 / MgCl 2 The preparation process of the composite carrier is the same as in Example 1, wherein MgCl 2 and SiO 2 The mass ratio is equal to 5; the specific synthesis steps of the loading substance i1 are the same as those of the loading substance e1 in Example 1. Elemental analysis of load i1: C, 33.66%; N, 1.67%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com