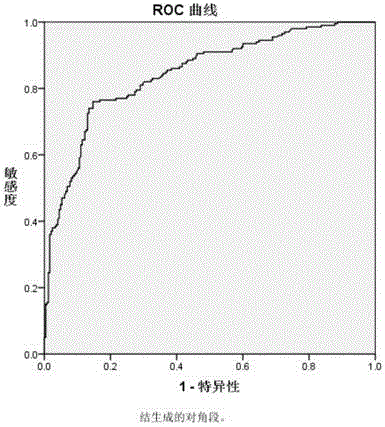
Time-resolved fluorescence immunoassay method of content of sH2a in serum, and detection kit thereof
A time-resolved, immunofluorescence technology, applied in the field of protein detection, can solve the problems of narrow linear range, long detection time, high detection background, and achieve the effect of wide linear range, short detection time and high accuracy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1, expression, purification and identification of sH2a recombinant protein
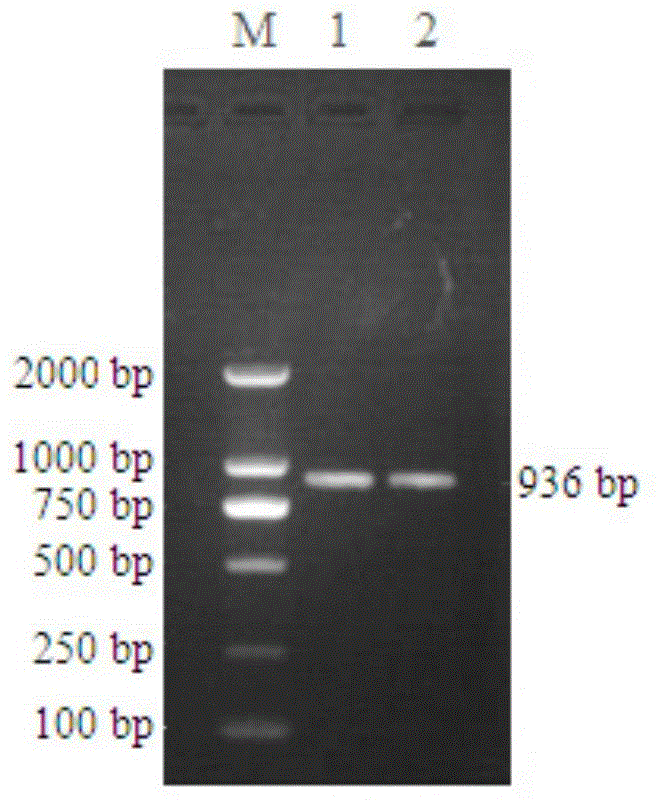
[0045] Using liver cancer tissue cDNA as template, 5'-CGGGATCCATGGCCAAGGACTTTCAAGATATCCA-3' (SEQ ID No.1) and 5'-CGGAATTCTCAGGCCACCTCGCCGGT-3' (SEQ ID No.2) as primers, PCR amplified sH2a gene fragment ( figure 1 ), after gel cutting, recovery and purification, it was connected to the cloning vector pCR2.1 and sequenced for identification, and then the sH2a gene fragment with the correct sequence identification was cloned into the expression vector pET28a(+), the restriction sites were BamH I and EcoR I, The recombinant plasmid pET28a-sH2a was obtained, which contained 6×HIS tag, which was convenient for subsequent protein purification.
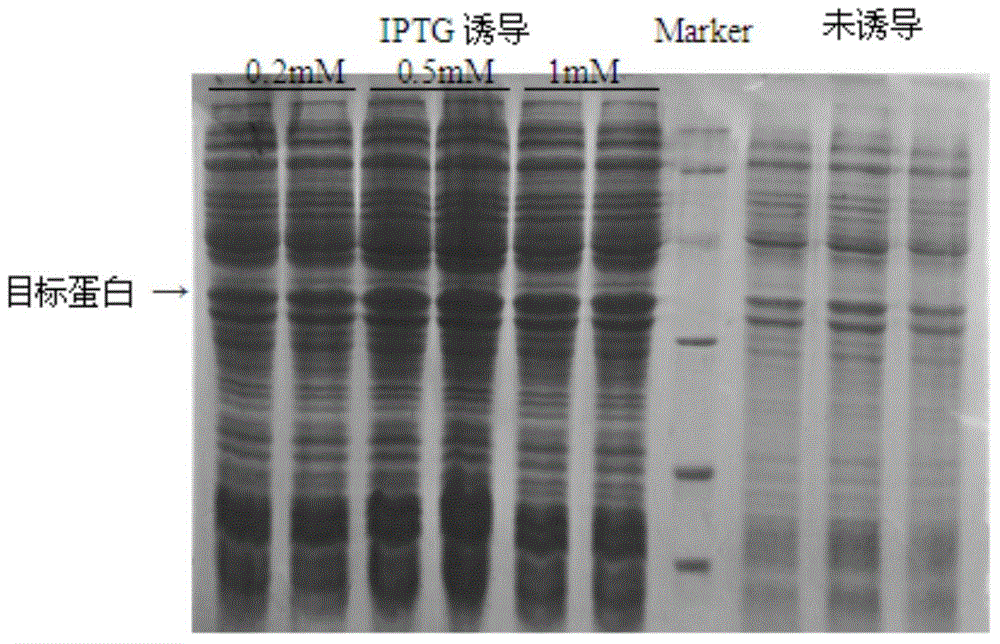
[0046] The recombinant plasmid pET28a-sH2a was transformed into Escherichia coli BL21(DE3), and the successfully transformed clones were picked and cultured at 37°C until the OD600nm value was about 0.6, and 0.2, 0.5, and 1 mM IPTG were added respec...
Embodiment 2
[0048] Embodiment 2, preparation of anti-sH2a monoclonal antibody
[0049] Two 6-week-old BALB / c female mice were taken and immunized according to the following steps:
[0050] First immunization: Mix the sH2a recombinant protein solution with Freund’s complete adjuvant at a volume ratio of 1:1, fully emulsify, and inject 0.1ml subcutaneously at two points on the back of the mouse (30 μg of sH2a recombinant protein per mouse);
[0051] The second immunization: after an interval of 14 days, mix the sH2a recombinant protein solution with Freund’s incomplete adjuvant at a volume ratio of 1:1, fully emulsify, and inject 0.1ml (sH2a recombinant Protein 50μg / piece);
[0052] The third immunization: after an interval of 14 days, mix the sH2a recombinant protein solution with Freund’s incomplete adjuvant at a volume ratio of 1:1, fully emulsify, and inject 0.1ml subcutaneously at the back of the shoulder and abdomen on both sides of the mouse (sH2a recombinant protein 50μg / piece); ...
Embodiment 3
[0057] Example 3, paired screening of anti-sH2a monoclonal antibodies
[0058] The above 9 strains of purified anti-sH2a monoclonal antibodies were respectively coated in solid-phase microwell plates, and the other 8 strains of anti-sH2a monoclonal antibodies labeled with Eu3+ except themselves were used for pairwise pairing experiments. The results showed that anti-sH2a monoclonal antibodies 2D4H5 and 4A7D8 could be paired to establish a reaction system (Table 2).
[0059] Table 2s Screening of H2a paired monoclonal antibodies (double-antibody sandwich method, coated with 2D4H5)
[0060]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com