Preparation for combined treatment of head and neck neoplasms, and uses thereof
A head and neck tumor and preparation technology, applied in the field of biomedicine, can solve problems such as undiscovered head and neck tumors, and achieve the effects of reducing treatment cost, enhancing curative effect, and reducing dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Preparation of human recombinant arginase.
[0050] The coding sequence of human arginase (NM_000045.3) was obtained by PCR method. The full-length coding sequence of the gene is 969bp, with restriction sites XhoI and EcoRI. Use XhoI and EcoRI endonucleases to double-digest the PCR product and the empty plasmid vector respectively, connect the target gene to the vector and transform the ampicillin resistance plate, pick several positive transformants, take one of them to amplify and extract the plasmid, and carry out XhoI And EcoRI endonuclease double digestion identification. The recombinant plasmid was extracted, the recombinant vector was digested with BglⅡ to linearize, and Pichia pastoris was transformed by electroporation. Pick positive transformants. Through RDB, MM and MD plate screening, clones with the phenotype of his+muts were obtained, and the expression was further induced on the shaker level, and the expression of arginase on the shaker leve...
Embodiment 2
[0054] Embodiment 2: the prescription of autophagy inhibitor drug
[0055] Chloroquine (CQ), purchased from sigma company;
[0056] Ammonium chloride (NH 4 Cl), purchased from sigma company;
[0057] Hydroxychloroquine, purchased from sigma company;
[0058] 3-methyladenine (3-MA), purchased from sigma company;
[0059] PI3K inhibitor LY294002, purchased from Cell Signaling Technology;
[0060] Bafilomycin A1 (Bafilomycin A1), purchased from sigma company;
[0061] (1) Preparation of chloroquine: get appropriate amount of chloroquine dissolved in pure water to make 10mmol / L stock solution, filter and sterilize with a filter of 0.1 μm and store at 4°C, use PRMI-1640 medium for in vitro experiments (Thermo Fisher) Seoul Technology Company) was diluted 500-1000 times to inhibit autophagy.
[0062] (2) Preparation of ammonium chloride: Dissolve an appropriate amount of ammonium chloride in water to prepare a 0.4 mol / L storage solution, filter and sterilize with a 0.1 μm filt...
Embodiment 3
[0068] Example 3: Human recombinant arginase can induce autophagy in laryngeal carcinoma Tu212 cells
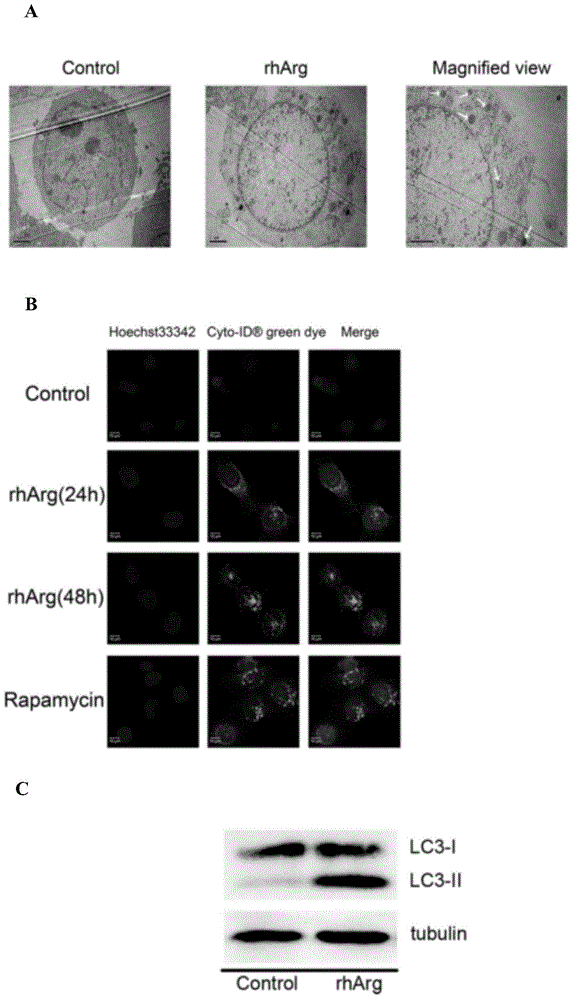
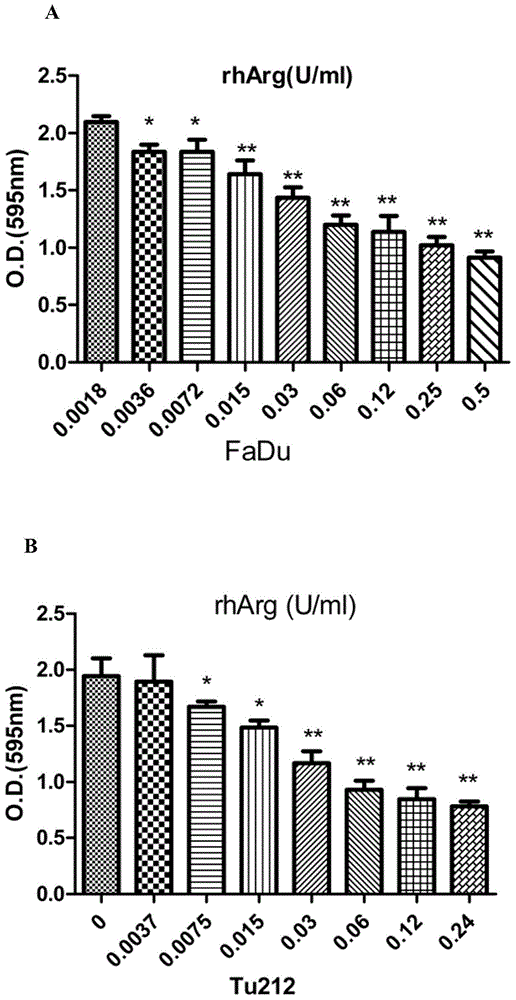
[0069] Laryngeal cancer Tu212 cells (purchased from the Cell Resource Center of the Central Laboratory of Hunan Xiangya Medical College) were treated with 0.12 U / ml arginase for 24 hours, then embedded in paraffin, sectioned, and stained, and the subunits of the cells were observed under a transmission electron microscope. Microstructure, the results are as figure 1 As shown in A, there are a large number of electron-dense inclusions in the cells of the administration group, and the typical double-membrane structure autophagosomes can be clearly seen after zooming in, while none were found in the control group.
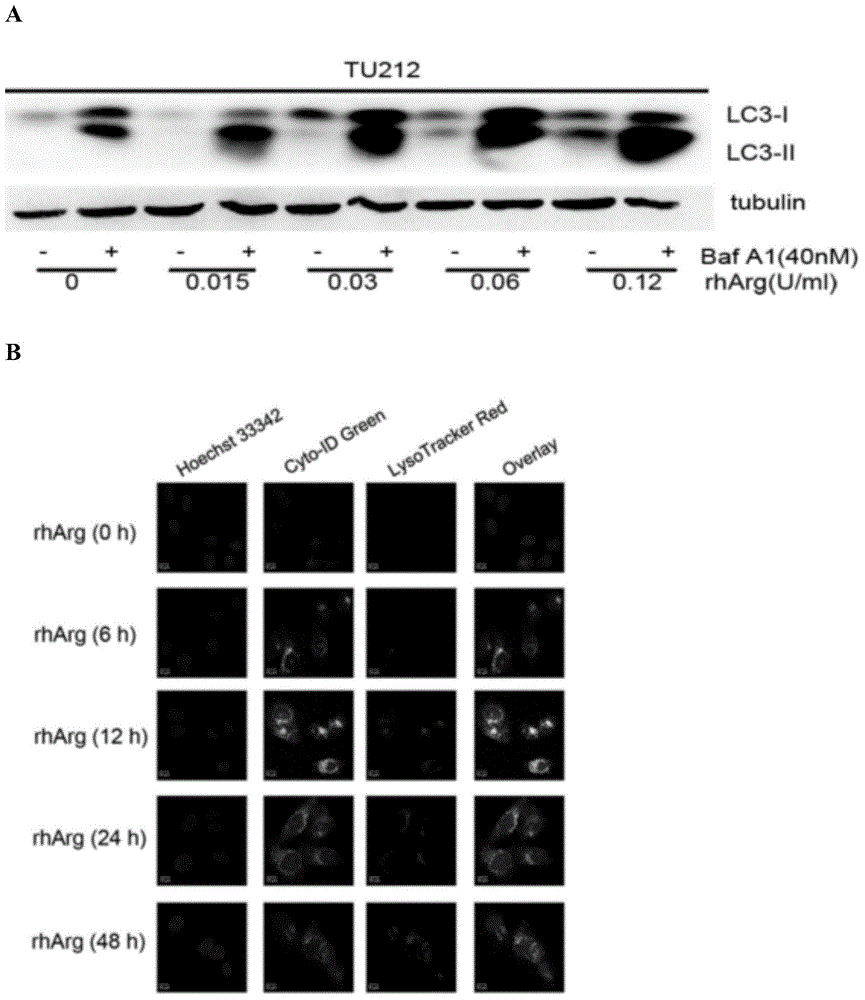
[0070] Laryngeal carcinoma Tu212 cells were treated with 0.12U / ml arginase for 24h and 48h, and Cyto- Autophagy Detection Kit Autophagosome Staining Kit (ENZO Life Science Research Institute) was processed according to the instructions, and then the green fluoresce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com