Pharmaceutical composition for treating infectious diseases and preparation thereof
A technology for infectious diseases and compositions, which is applied in the field of compositions of cefazedone sodium for injection, can solve the problems of high content of related substances, affecting drug safety, high content of substances, etc., and achieves improved stability, good fluidity, good safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Preparation of Cefoxizone Sodium Crystals
[0026] Prepare 30 DEG C of cefazedone sodium crude product saturated aqueous solution, then add the mixed solvent of n-butanol, acetone and sherwood oil of saturated solution volume 30%, the volume ratio of the mixed solvent of described n-butanol, acetone and sherwood oil is 4:8:12, after stirring evenly, stir while cooling down, the cooling rate is 5-10°C / hour, and the stirring speed is 100-110 rpm After ℃, the stirring was stopped, and the crystals were left to grow for 3 hours, filtered, and dried under reduced pressure at 35 ℃ for 6 hours to obtain cefazedone sodium crystals.
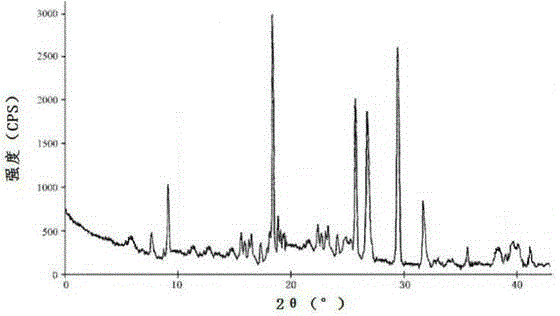
[0027] The X-ray powder diffraction pattern obtained by the cefazedone sodium crystal prepared using Cu-Kα ray measurement is as follows figure 1 As shown, the main particle size of the cefazedone sodium crystal is 40-50 μm, and the distribution width is 25-65 μm through scanning electron microscope observation and particle size analy...
Embodiment 2
[0028] Example 2: Preparation of Cefazedone Sodium Composition
[0029] The composition comprises: 1 part by weight of cefazedone sodium crystal prepared by the present invention, and 0.3 part by weight of anhydrous sodium carbonate.
[0030] The preparation method is:
[0031] (1) Weigh cefazedone sodium crystals and anhydrous sodium carbonate in proportion, and mix them thoroughly;
[0032] (2) Dispense into sterilized vials and stopper them.
Embodiment 3
[0033] Example 3: Preparation of Cefazedone Sodium Composition
[0034] The composition comprises: 1 part by weight of the cefazedone sodium crystal prepared by the present invention, and 0.5 part by weight of anhydrous sodium carbonate.
[0035] The preparation method is:
[0036] (1) Weigh cefazedone sodium crystals and anhydrous sodium carbonate in proportion, and mix them thoroughly;
[0037] (2) Dispense into sterilized vials and stopper them.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Master granularity | aaaaa | aaaaa |
| Distribution width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com