Preparation method of catalyst for gas phase fluorination reaction
A gas-phase fluorination and catalyst technology, applied in physical/chemical process catalysts, chemical instruments and methods, halogen substitution preparation, etc., can solve the problems of difficulty in improving the activity, easy carbon deposition catalyst life, and production benefits that are not worth the gain, and achieve high response Active and selective effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
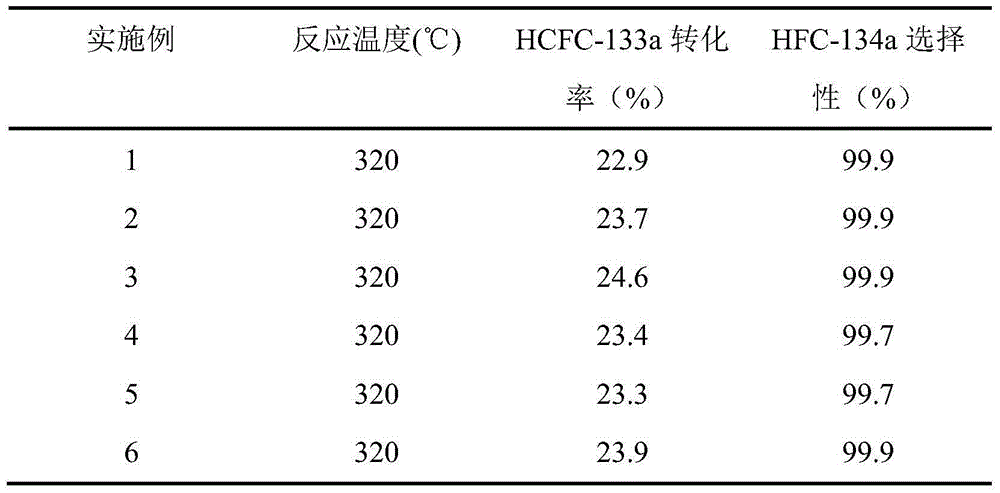
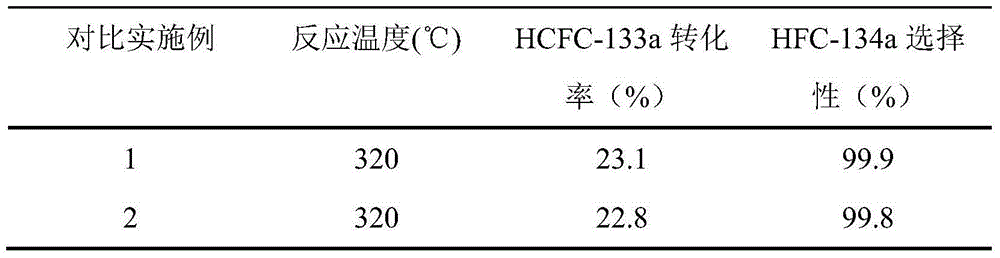
Examples
Embodiment 1
[0022] ① According to the total mass ratio of SBA-15 template agent to chromium nitrate nonahydrate and sucrose is 1:4, the mass ratio of chromium nitrate nonahydrate and sucrose is 100:3. Weigh the SBA-15 template agent, chromium nitrate nonahydrate and sucrose respectively. Mix chromium nitrate nonahydrate, sucrose and deionized water evenly to prepare a solution, then add the mixed solution to SBA-15, stir at room temperature for 4 hours, and then heat in a water bath to remove water;
[0023] ②After drying in an oven at 100°C for 12 hours, then calcined at 600°C for 4 hours in a nitrogen atmosphere, and then calcined in an air atmosphere at 450°C for 4 hours, and the carbon was burned to obtain Cr filled 2 o 3 SBA-15;
[0024] ③According to La 2 o 3 The mass percentage is the filled Cr obtained in ② 2 o 3 0.1% of SBA-15, weighed La(NO 3 ) 3 ·6H 2 O, add distilled water, stir to dissolve, form a solution, add to the filled Cr obtained in ② 2 o 3 Immerse in SBA-15...
Embodiment 2
[0027] ① According to the total mass ratio of SBA-15 template agent to chromium nitrate nonahydrate and sucrose is 1:4, the mass ratio of chromium nitrate nonahydrate and sucrose is 100:5. Weigh the SBA-15 template agent, chromium nitrate nonahydrate and sucrose respectively. Mix chromium nitrate nonahydrate, sucrose and deionized water evenly to prepare a solution, then add the mixed solution to SBA-15, stir at room temperature for 4 hours, and then heat in a water bath to remove water;
[0028] ②After drying in an oven at 100°C for 12 hours, then calcined at 600°C for 4 hours in a nitrogen atmosphere, and then calcined in an air atmosphere at 450°C for 4 hours to obtain Cr-filled 2 o 3 SBA-15;
[0029] ③According to La 2 o 3 The mass percentage is the filled Cr obtained in ② 2 o 3 0.2% of SBA-15, weighed La(NO 3 ) 3 ·6H 2 O, add distilled water, stir to dissolve, form a solution, add to the filled Cr obtained in ② 2 o 3 Immerse in SBA-15 for 4 hours, dry at 100°C,...
Embodiment 3
[0032]① According to the total mass ratio of SBA-15 template agent to chromium nitrate nonahydrate and sucrose as 1:4, the mass ratio of chromium nitrate nonahydrate and sucrose as 100:8. Weigh the SBA-15 template agent, chromium nitrate nonahydrate and sucrose respectively. Mix chromium nitrate nonahydrate, sucrose and deionized water evenly to prepare a solution, then add the mixed solution to SBA-15, stir at room temperature for 4 hours, and then heat in a water bath to remove water;
[0033] ②After drying in an oven at 100°C for 12 hours, then calcined at 600°C for 4 hours in a nitrogen atmosphere, and then calcined in an air atmosphere at 450°C for 4 hours to obtain Cr-filled 2 o 3 SBA-15;
[0034] ③According to La 2 o 3 The mass percentage is the filled Cr obtained in ② 2 o 3 0.3% of SBA-15, weighed La(NO 3 ) 3 ·6H 2 O, add distilled water, stir to dissolve, form a solution, add to the filled Cr obtained in ② 2 o 3 Immerse in SBA-15 for 4 hours, dry at 100°C, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com