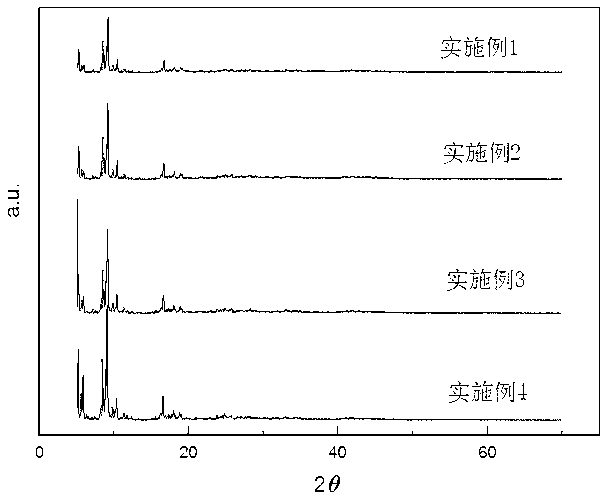
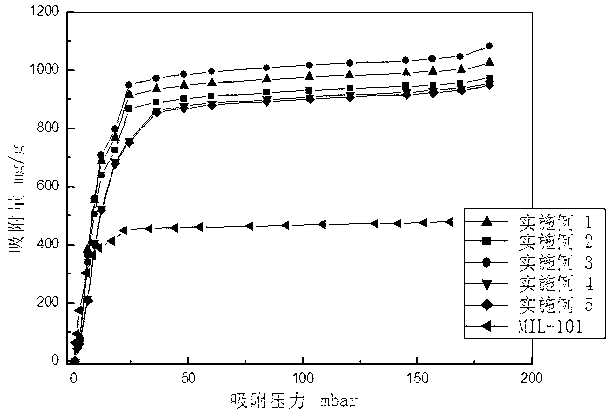
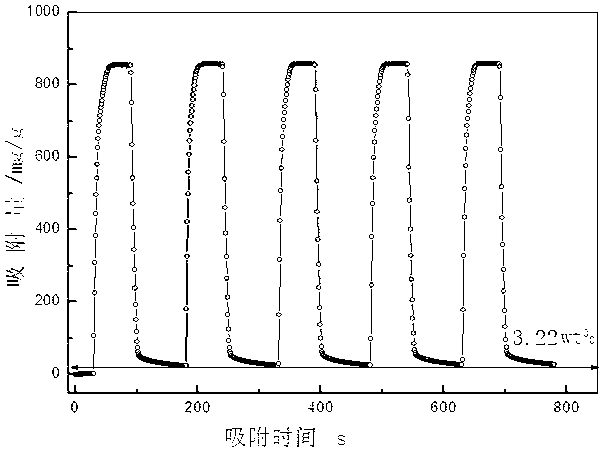
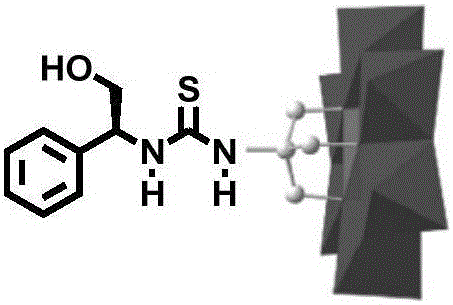
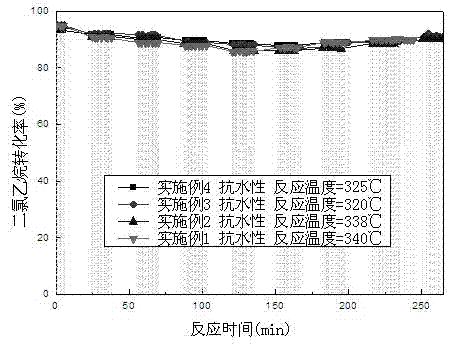
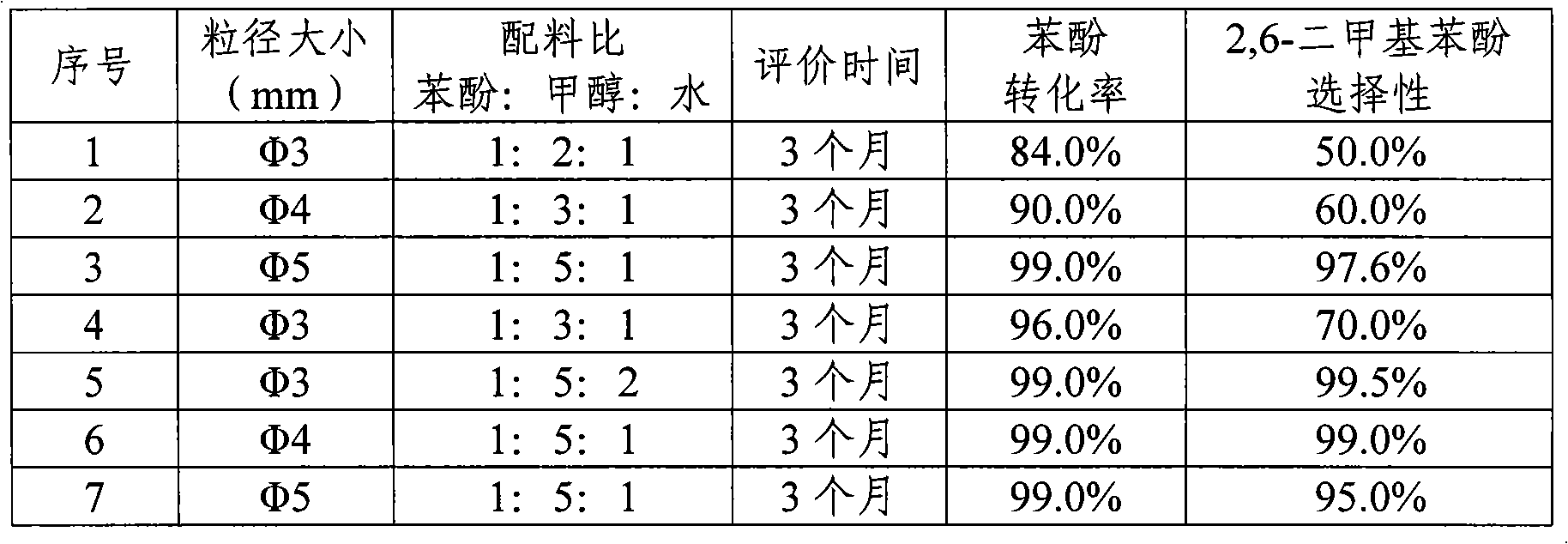
Patents
Literature
132 results about "Chromium nitrate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chromium(III) nitrate describes several inorganic compounds consisting of chromium, nitrate and varying amounts of water. Most common is the dark violet hydrated solid, but an anhydrous green form is also known. Chromium(III) nitrate compounds are of a limited importance commercially, finding some applications in the dyeing industry. It is common in academic laboratories for the synthesis of chromium coordination complexes.
Long-afterglow nanomaterial based on ion doping as well as preparation method and application of long-afterglow nanomaterial
InactiveCN105754595AAbundant and easy to get raw materialsThe synthesis method is simpleIn-vivo testing preparationsLuminescent compositionsZinc nitrateChromium nitrate
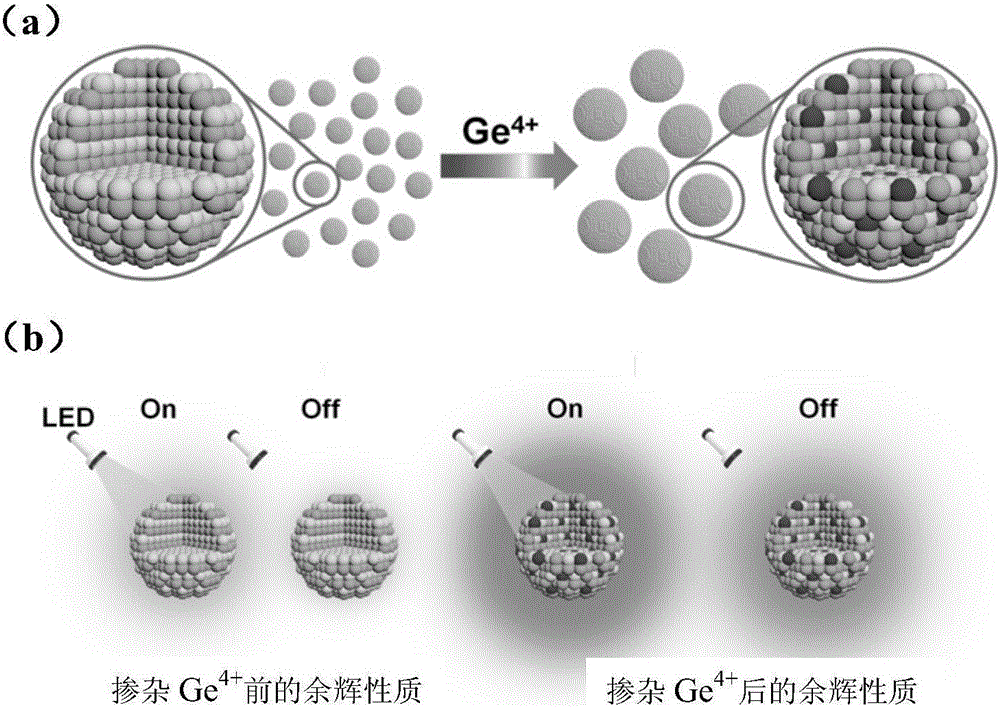
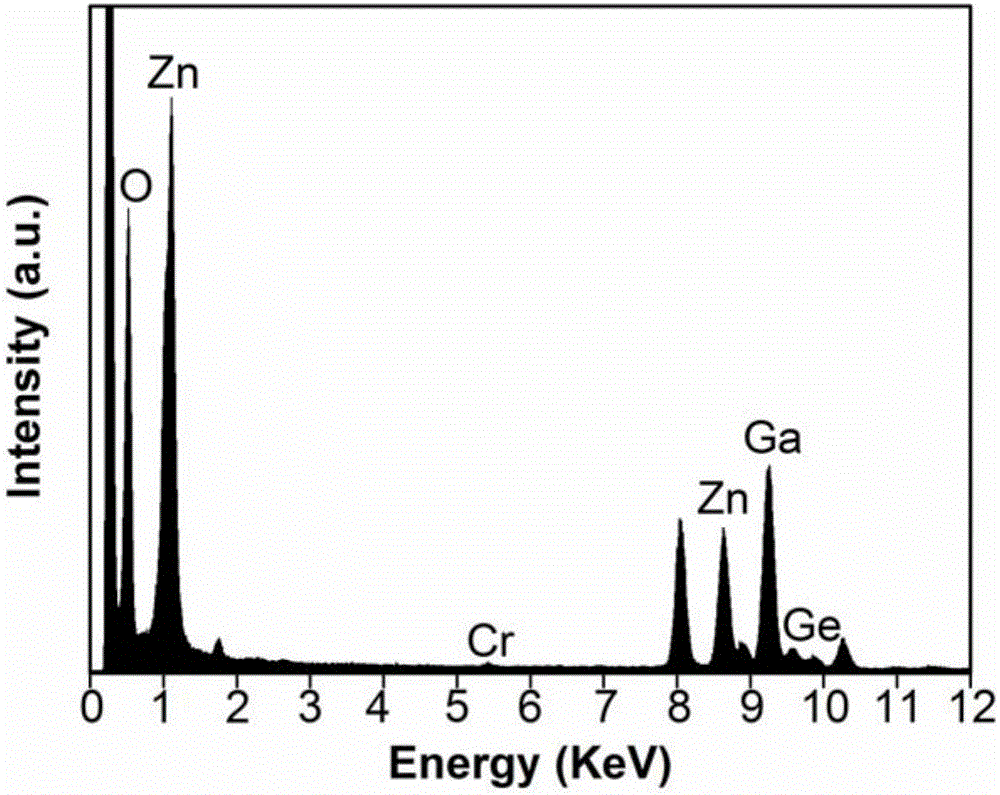
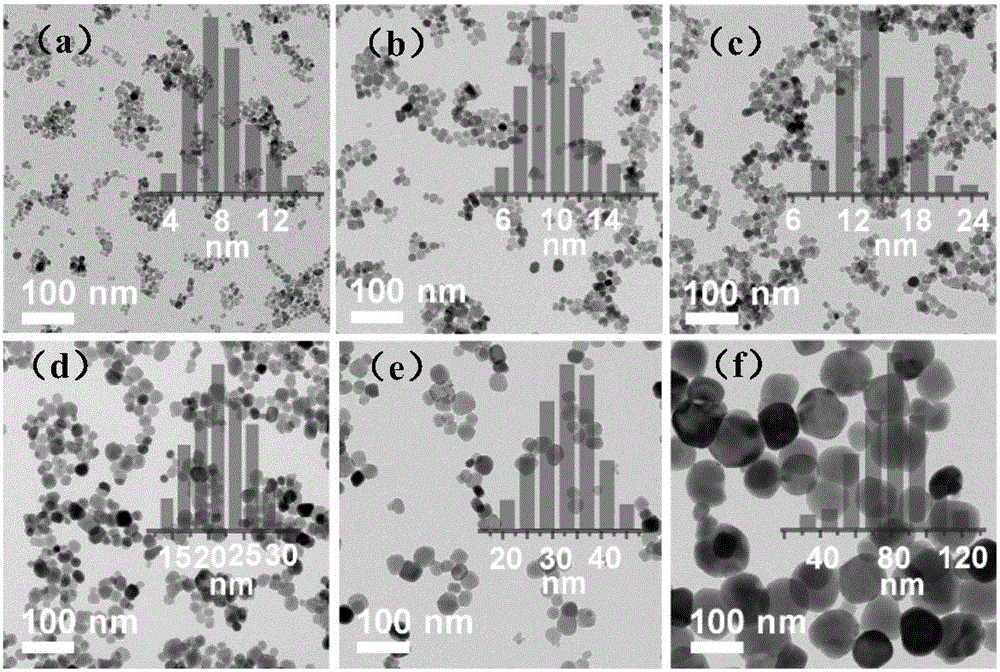
The invention discloses an afterglow nanomaterial and a preparation method of a long-afterglow nanomaterial with sizes and spectrum adjusted on basis of ion doping. An expression formula of the nanomaterial is Zn(1+x)Ga(2-2x)GexO4:0.75%Cr, wherein x is larger than or equal to 0 and smaller than or equal to 0.5, and the particle size is 7 nm-80 nm. According to the preparation method, a zinc nitrate solution, a gallium nitrate solution, a sodium germinate solution and a chromium nitrate solution in specific proportions are mixed together, ammonia water is added rapidly while the mixture is stirred, and the pH of the mixed solution is adjusted to 10; then, the mixed solution is transferred to a high-temperature hydrothermal kettle and reacts at the temperature of 120 DEG C, and the afterglow nanomaterial is obtained. The method is simple and easy to implement, severe experiment conditions and complicated large instruments are not required, synthesized nanoparticles are uniform in sizes and have a good water-phase dispersion property and high afterglow strength, the afterglow time can reach 10 h, and accordingly, the synthesized nanoparticles are suitable for improving the physical and chemical properties of the long-afterglow nanomaterial.
Owner:WUHAN UNIV
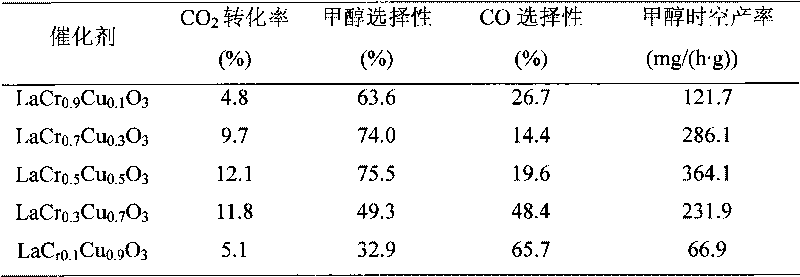
Catalyst for synthesizing methanol by direct hydrogenation of carbon dioxide and preparation method thereof
InactiveCN101690894AImprove performanceOvercome the disadvantages of low conversion rate and poor selectivity of methanolOrganic compound preparationHydroxy compound preparationCopper nitrateHYDROSOL
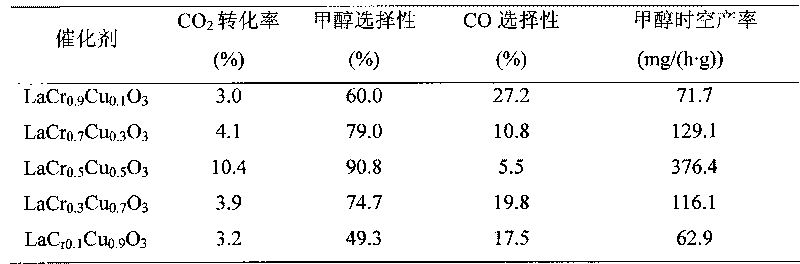
The invention discloses a catalyst for synthesizing methanol by direct hydrogenation of carbon dioxide and a preparation method thereof and relates to the catalyst. The invention provides the catalyst for synthesizing the methanol by direct hydrogenation of the carbon dioxide and the preparation method thereof. The catalyst is LaCr1-xCuxO3, wherein x is the relative mole fraction of a main component of Cu, x is equal to 0.1-0.9, and La: Cr: Cu is equal to 1: (1-x): x. The preparation method comprises the steps of preparing copper nitrate, chromium nitrate, lanthanum nitrate and citric acid into water solution, forming a sol under the radiation of an infrared lamp, heating, decomposing nitrogen oxides and organic acids, pre-baking in an atmosphere furnace at the temperature of 350-450 DEG C for 2-4h, baking at the temperature of 700-800 DEG C, and obtaining the catalyst for synthesizing the methanol by direction hydrogenation of the carbon dioxide. The citric acid complexation-rapid combustion method is adopted for overcoming the disadvantages of lower conversion rate, poorer selectivity of the methanol and the like during the catalysis of the carbon dioxide by using the existing catalyst for synthesizing the methanol by hydrogenation of the carbon dioxide, and the prepared catalyst has stable performances.
Owner:XIAMEN UNIV
Metal organic skeleton-graphite oxide nano composite adsorption material and preparing method thereof
ActiveCN102335592ALarge specific surface areaHigh porosityOther chemical processesMetal-organic frameworkChromium nitrate
The invention discloses a metal organic skeleton-graphite oxide nano composite adsorption material and a preparing method thereof. The material is composed of graphite oxide and chromium-based metal organic skeleton. The preparing method of the material comprises the following steps of: dissolving chromium nitrate and terephthalic acid in deionized water, stirring and simultaneously adding hydrofluoric acid dropwise, then adding graphite oxide and uniformly stirring to obtain reaction liquid, and conducting hydro-thermal reaction under program temperature controlling; and after sequentially conducting washing with water and washing with ethanol for the decomposed materials, centrifuging, drying and obtaining the metal organic skeleton-graphite oxide nano composite adsorption material. Theadsorption capacity of the adsorption material for hydrocarbon volatile organic matters is greatly increased, and the adsorption material is easy for desorption and regeneration. The preparing methodhas simple process and low cost.
Owner:SOUTH CHINA UNIV OF TECH
Preparation method of hydrophobic MIL-101 chromium organometallic skeleton material
ActiveCN102408447ALangmuir high surface areaLarge adsorption capacityOther chemical processesDispersed particle separationChromium nitrateMaterials science
The invention discloses a preparation method of a hydrophobic MIL-101 chromium organometallic skeleton material. The method comprises the following steps: dissolving chromium nitrate and 1,4-naphthalenedicarboxylic acid in water, adding hydrofluoric acid and acetic acid to stir evenly, performing a hydrothermal reaction at 210-220 DEG C, washing the obtained product with deionized water, dimethylformamide and ethanol in turn, centrifuging, filtering, and drying to prepare the hydrophobic MIL-101 chromium organometallic skeleton material. The hydrophobic MIL-101 chromium organometallic skeleton material has high Langmuir surface area and also has the high specific surface area and micro-mesoporous skeleton of a traditional MIL-101 material; and as a benzene ring is grafted on the organic connector in the skeleton, the material has higher hydrophobicity and the adsorption capacity of the material to nonpolar organic volatile substances can be increased.
Owner:SOUTH CHINA UNIV OF TECH
Six-element high-entropy oxide material for lithium ion battery and preparation method
ActiveCN110556536AGuaranteed stabilityImprove cycle stabilityCell electrodesSodium-ion batteryChromium nitrate
Owner:ANHUI UNIVERSITY OF TECHNOLOGY
Aqueous solution of chromium salt and method for producing same
InactiveUS20070086938A1Overcome disadvantagesPigmenting treatmentNitrogen compoundsOXALIC ACID DIHYDRATEChloride
Disclosed is an aqueous solution of a chromium salt, in which the oxalic acid content is 8% by weight or less relative to chromium. In the aqueous solution of the chromium salt, the total organic carbon content is 4% by weight or less relative to chromium. The chromium salt is preferably a chromium chloride, a chromium phosphate, or a chromium nitrate. The chromium chloride preferably contains a basic chromium chloride represented by the composition formula Cr(OH)xCly (wherein 0<x≦2, 1≦y<3, and x+y=3). The chromium phosphate is preferably one represented by the composition formula Cr(H3−3 / nPO4)n (wherein n is a number satisfying 2≦n≦3). The chromium nitrate is preferably a basic chromium nitrate represented by the composition formula Cr(OH)x(NO3)y (wherein 0<x≦2, 1≦y<3, and x+y=3).
Owner:NIPPON CHECMICAL IND CO LTD
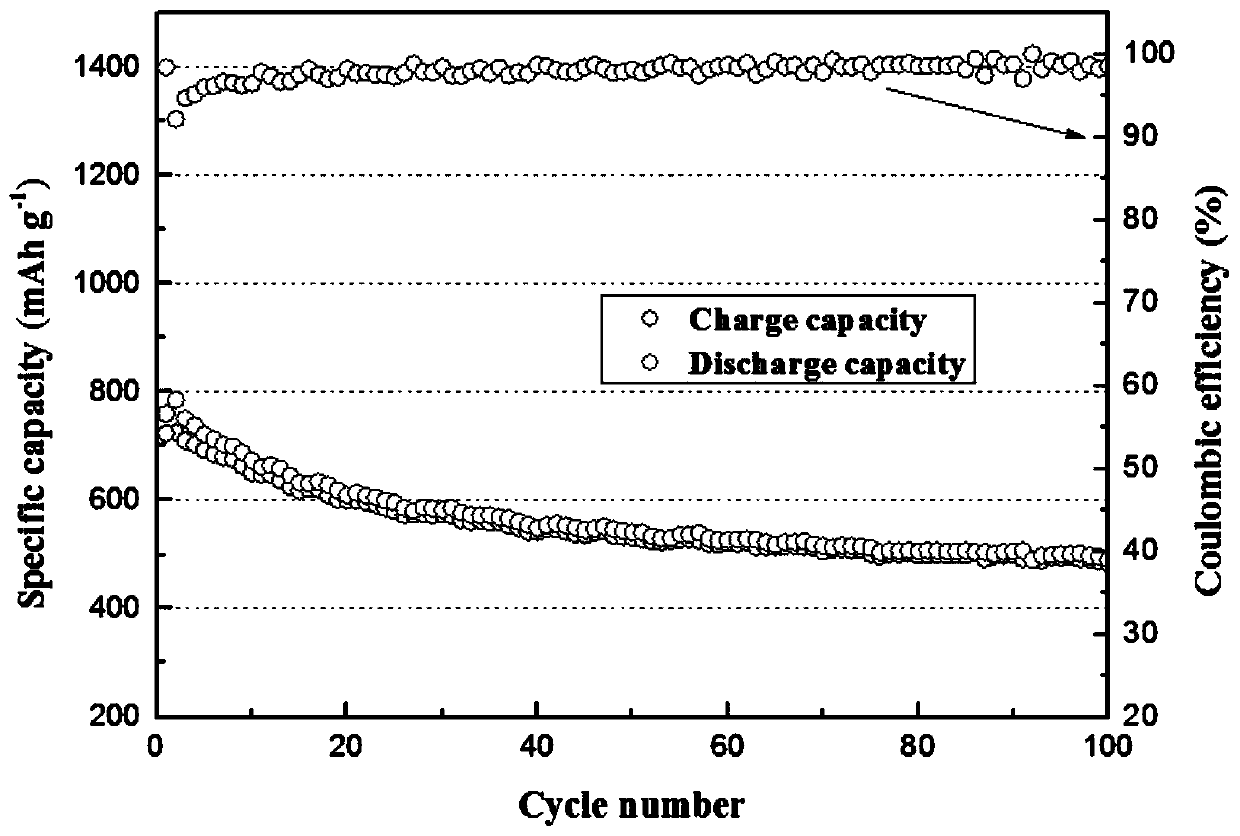
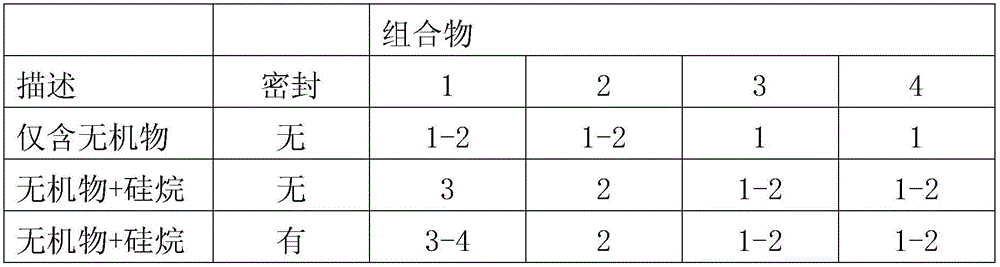
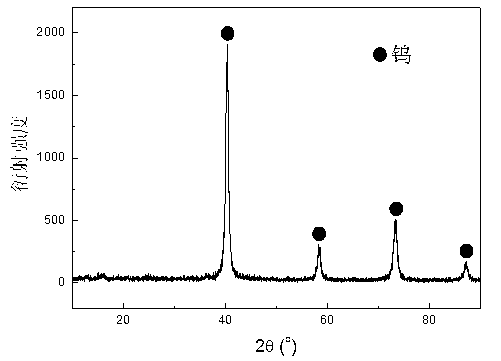
Preparation method of nano tungsten carbide
A preparation method of nano tungsten carbide comprises the following steps: (1) dissolving ammonium metatungstate, chromium nitrate and a water-soluble carbon source substance into in heated deionized water, sufficiently mixing, and performing spray drying, wherein the weight percentage of the water-soluble carbon source substance is 10-30%, the weight percentage of the chromium nitrate is 0.5-2% and the temperature of the deionized water is 70 DEG C; (2) performing auxiliary hydrogen reduction on powdery carbon obtained in the step (1), and before discharging the powder out of a furnace, passivating the powder with an inert gas, wherein the temperature is 710-850 DEG C, the heating rate is 10-15 DEG C / min and the reaction time is 2-5h; (3) annealing tungsten powder obtained in the step (2) at high temperature, wherein the annealing temperature is 1000-1300 DEG C and the annealing time is 1-3h; and (4) carbonizing the tungsten powder obtained in the step (3), and before discharging the powder out of the furnace, passivating the powder with the inert gas, wherein the carbonizing ratio is 6.21wt.%, hydrogen is used as a protective atmosphere, the carbonizing temperature is 1100-1400 DEG C and the carbonizing time is 1-4h. The particle size of the prepared tungsten carbide is 60-90nm; after the prepared tungsten carbide is crushed, agglomeration-free nano tungsten carbide powder can be obtained; the environment is polluted; and the development of a nanocrystalline WC-Co hard alloy can be effectively promoted.
Owner:NANCHANG UNIV
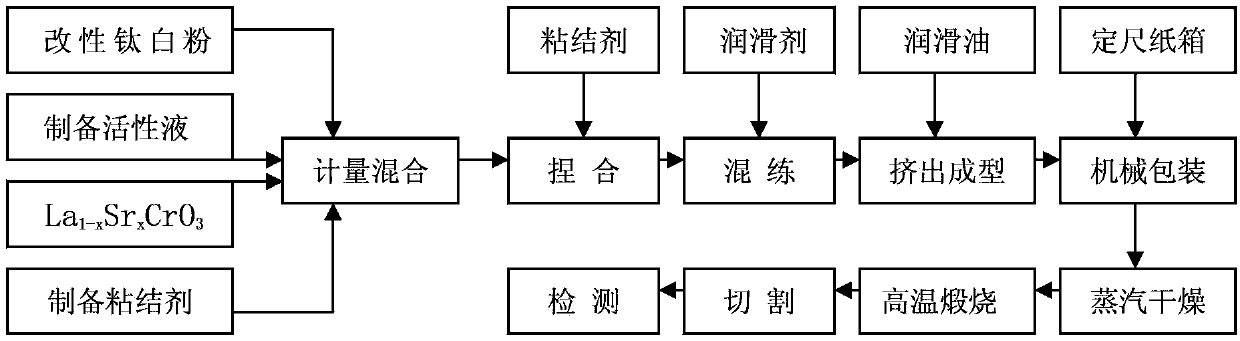
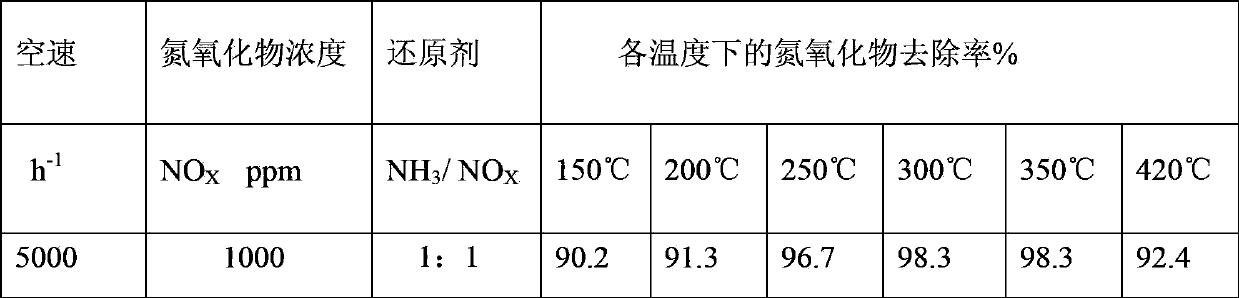
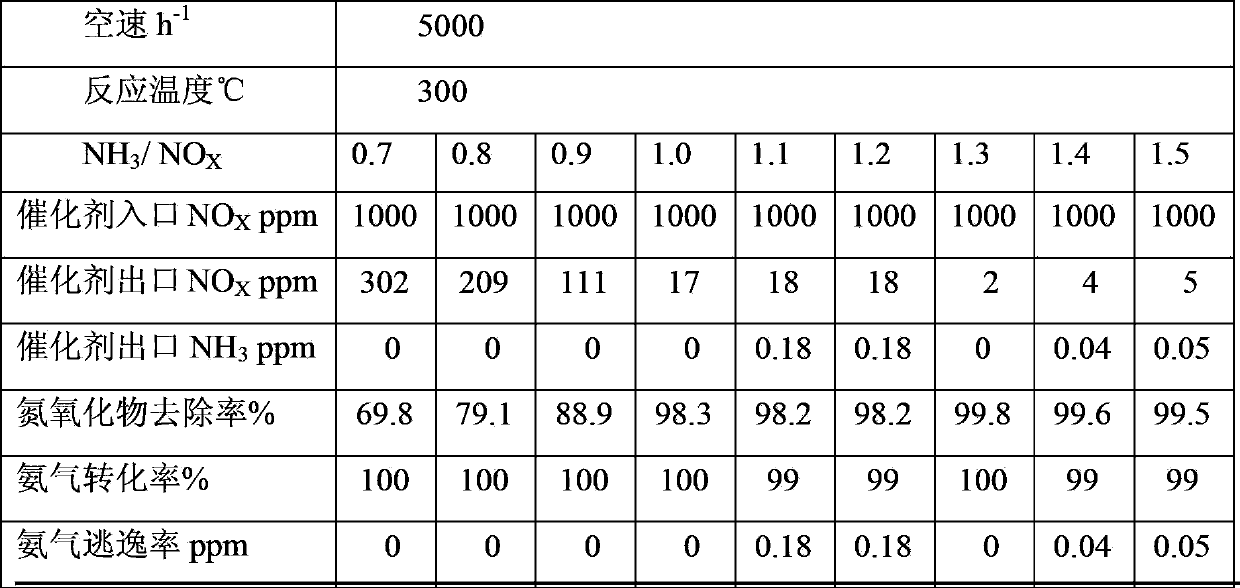
Perovskite-type substance La<1-x>Sr<x>CrO<3>, heat-engine plant denitration composite catalyst, and preparation methods of perovskite-type substance La<1-x>Sr<x>CrO<3> and heat-engine plant denitration composite catalyst
InactiveCN103861581AImprove performanceUltra-fine crystal shape lowDispersed particle separationMetal/metal-oxides/metal-hydroxide catalystsPhosphateHoneycomb like
The invention discloses perovskite-type substance La<1-x>SrCrO<3>, a heat-engine plant denitration composite catalyst, and preparation methods of the perovskite-type substance La<1-x>SrCrO<3> and the heat-engine plant denitration composite catalyst, and belongs to the technical field of flue gas denitrification catalyst. The preparation method of the perovskite-type substance La<1-x>SrCrO<3> is used for solving a problem that existing preparation method of the perovskite-type substance is complex. According to the preparation method of the perovskite-type substance La<1-x>SrCrO<3>, lanthanum nitrate, strontium nitrate, and chromium nitrate are taken as raw material precursors, and are dissolved in water, and then are subjected to high-temperature roasting so as to obtain finished products. The preparation methods of the heat-engine plant denitration composite catalyst is used for solving a problem of existing denitration catalyst that ammonia is released in denitration processes. According to the preparation methods of the heat-engine plant denitration composite catalyst, a titanium dioxide mixture, an active liquid, water, glycerin, ethylene glycol, glass fiber, and aluminium dihydrogen phosphate are subjected to kneading so as to obtain ceramic clay; the ceramic clay is subjected to vacuum extrusion so as to obtain honeycomb ceramic blank; and the honeycomb ceramic blank is subjected to steam drying and roasting so as to obtain products. The heat-engine plant denitration composite catalyst is high in denitration rate, and is capable of solving a problem of ammonia escaping.
Owner:SHANGHAI LANGT ELECTRIC POWER ENVIRONMENTAL PROTECTION TECH +1
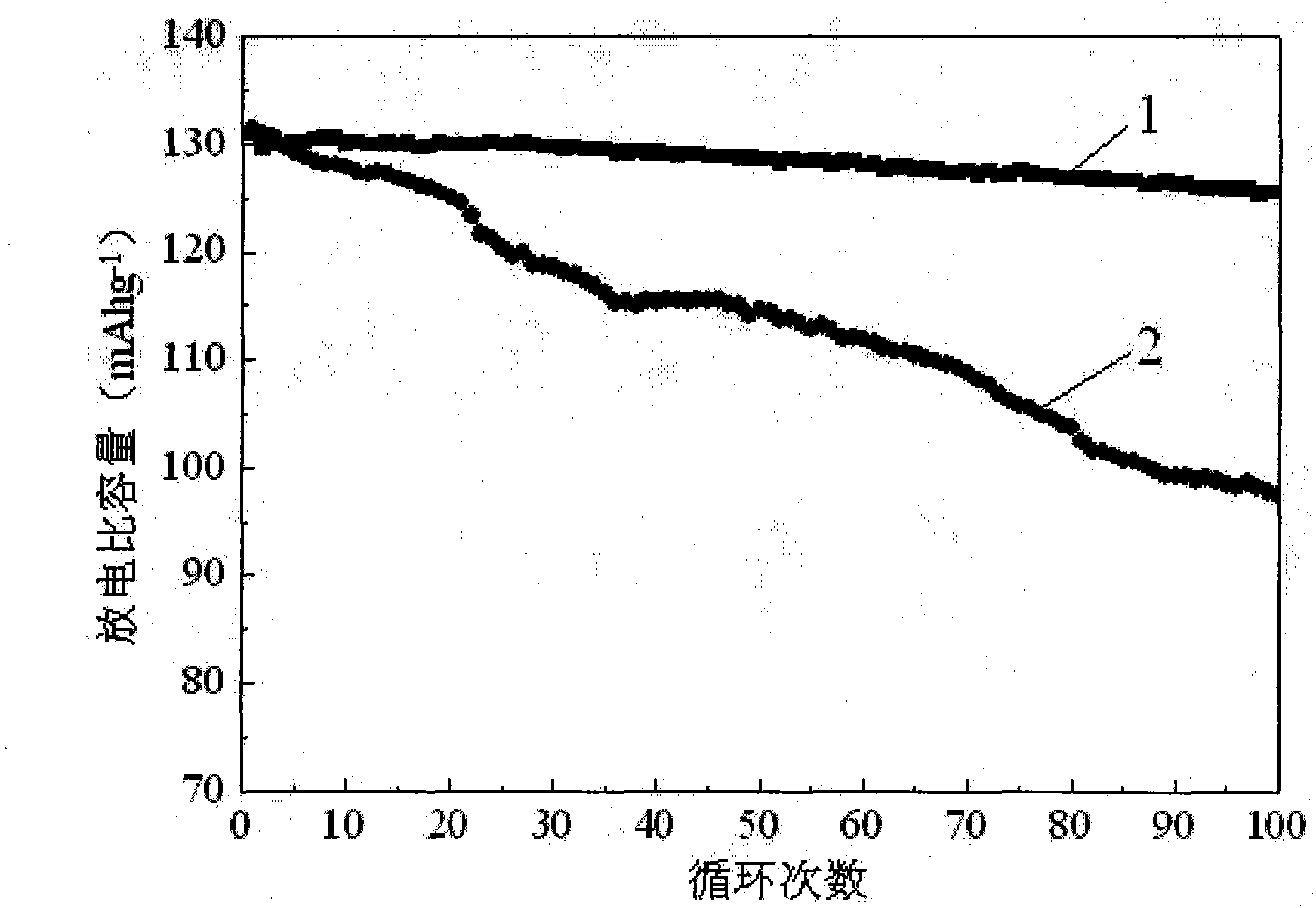
Method for improving electrochemical performance of positive material LiNi0.5Mn1.5O4 of lithium ion battery
InactiveCN101859895AReduce internal volumeImprove electrochemical performanceCell electrodesElectricityFree cooling
The invention discloses a method for improving the electrochemical performance of a positive material LiNi0.5Mn1.5O4 of a lithium ion battery, and belongs to the field of the positive material of the lithium ion battery. The method reduces the using amount of Cr<3+> for improving the electrochemical performance of LiNi0.5Mn1.5O4 by a conventional bulk phase doping method, reduces the environmental pollution and the human health hazard caused thereby and eliminates the hidden trouble of striping of a covering layer existing in a covering modification method. The method comprises the following steps of: dissolving chromium nitrate in aqueous solution of ethanol; adding LiNi0.5Mn1.5O4 into the solution and dispersing and stirring the mixture until the liquid phase is eliminated; and calcining the mixture and naturally cooling the mixture to obtain the positive material LiNi0.5Mn1.5O4 of the lithium ion battery with high electrochemical performance. The method improves the electrochemical performance of the positive material LiNi0.5Mn1.5O4 of the lithium ion battery, reduces the using amount of the chromium salt, ensures no obvious boundary between the doped Cr<3+> layer and the body of the positive material, reduces the environmental pollution, and also reduces the hazard to the human health.
Owner:HARBIN INST OF TECH
Preparation method for polyacid @ MIL-101 composite material and application of polyacid @ MIL-101 composite material
InactiveCN103769036AStable structureSimple methodOther chemical processesWater/sewage treatment by sorptionTetramethylammonium hydroxideDye absorption
The invention belongs to the technical field of inorganic composite materials and particularly relates to a preparation method for an MIL-101 composite material based on Keggin type poly-tungstate and a molecule-group porous material, and an application of dye absorption of the MIL-101 composite material. The material is prepared from the Keggin type poly-tungstate, tetramethylammonium hydroxide, terephthalic acid and chromic nitrate which are used as raw materials by adopting a hydrothermal method. The prepared material can be used for adsorbing a cation organic dye; the adsorption rate to methylene blue within short time can be up to 98% and the adsorption effect is obviously higher than that of a pure MIL-101 material and is higher than the adsorption capability of active carbon. The preparation method has the characteristics of being easy and convenient to prepare and rapid and efficient in adsorption; the prepared material has a wide acid-base applicable range, is easy to separate and can be repeatedly used.
Owner:NORTHEAST NORMAL UNIVERSITY
Method for chemical conversion treatment of the surface of aluminum material and aluminum material
Disclosed is a method for chemical conversion treatment of the surface of an aluminum material comprising aluminum or an aluminum alloy. The method comprises performing a chemical conversion treatment of the surface of the aluminum material with a treatment solution for 1 to 5 minutes to form a coating film having excellent corrosion resistance and coating film adhesion property, wherein the treatment solution contains a trivalent chromium, and has a chromium nitrate content of 3 to 12 g / L in terms of Cr(NO3)3, a ammonium vanadate content of 0.5 to 7 g / L in terms of NH4VO3, a potassium fluorotitanate content of 0.5 to 3 g / L in terms of K2TiF6, a lithium fluoride content of 0.5 to 3 g / L in terms of LiF, a nitric acid content of 0.5 to 1.8 mL / L in terms of HNO3, and a liquid temperature of 40 to 50 C. Also disclosed is an aluminum material having a coating film formed by the method.
Owner:MITSUI MINING & SMELTING CO LTD
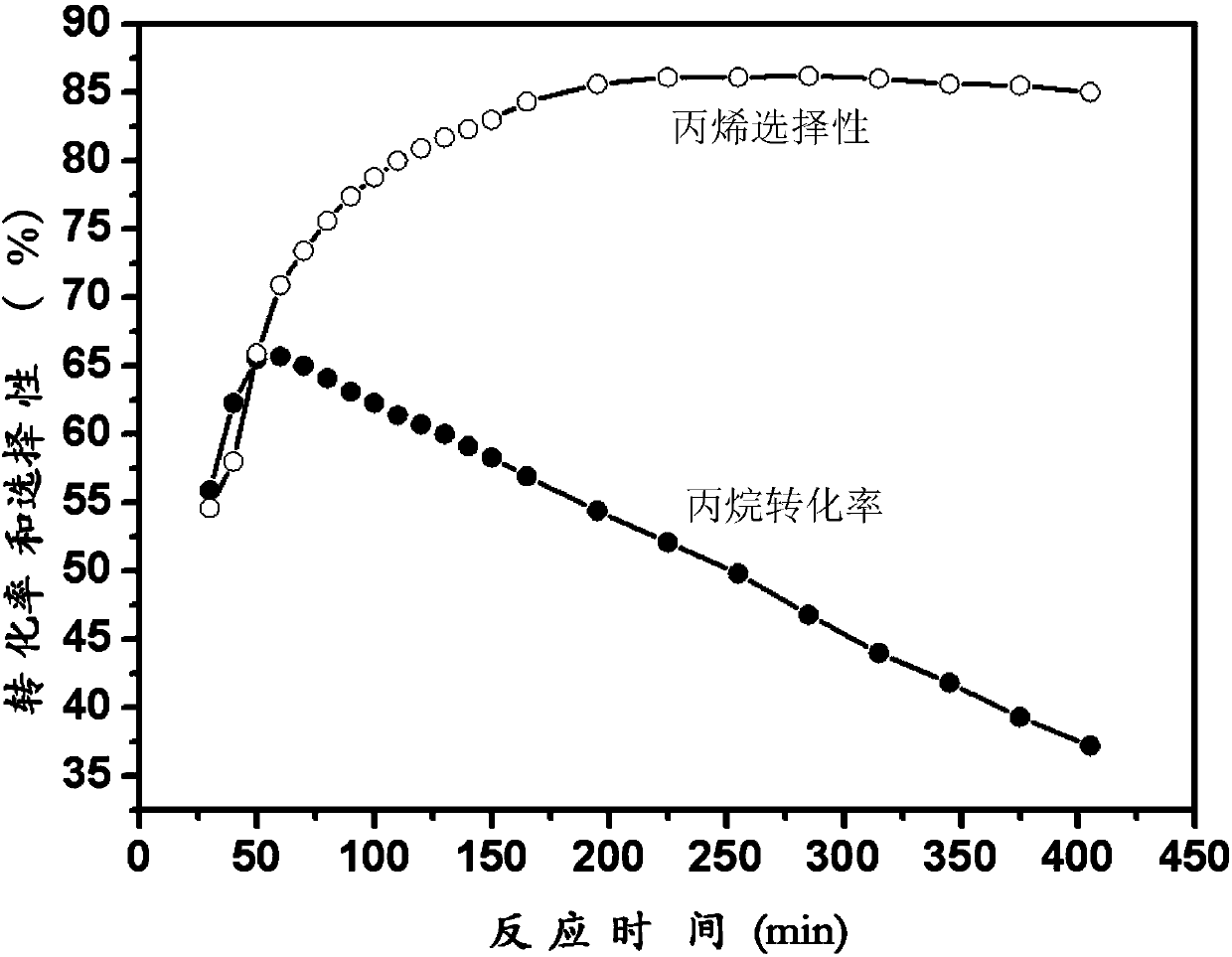
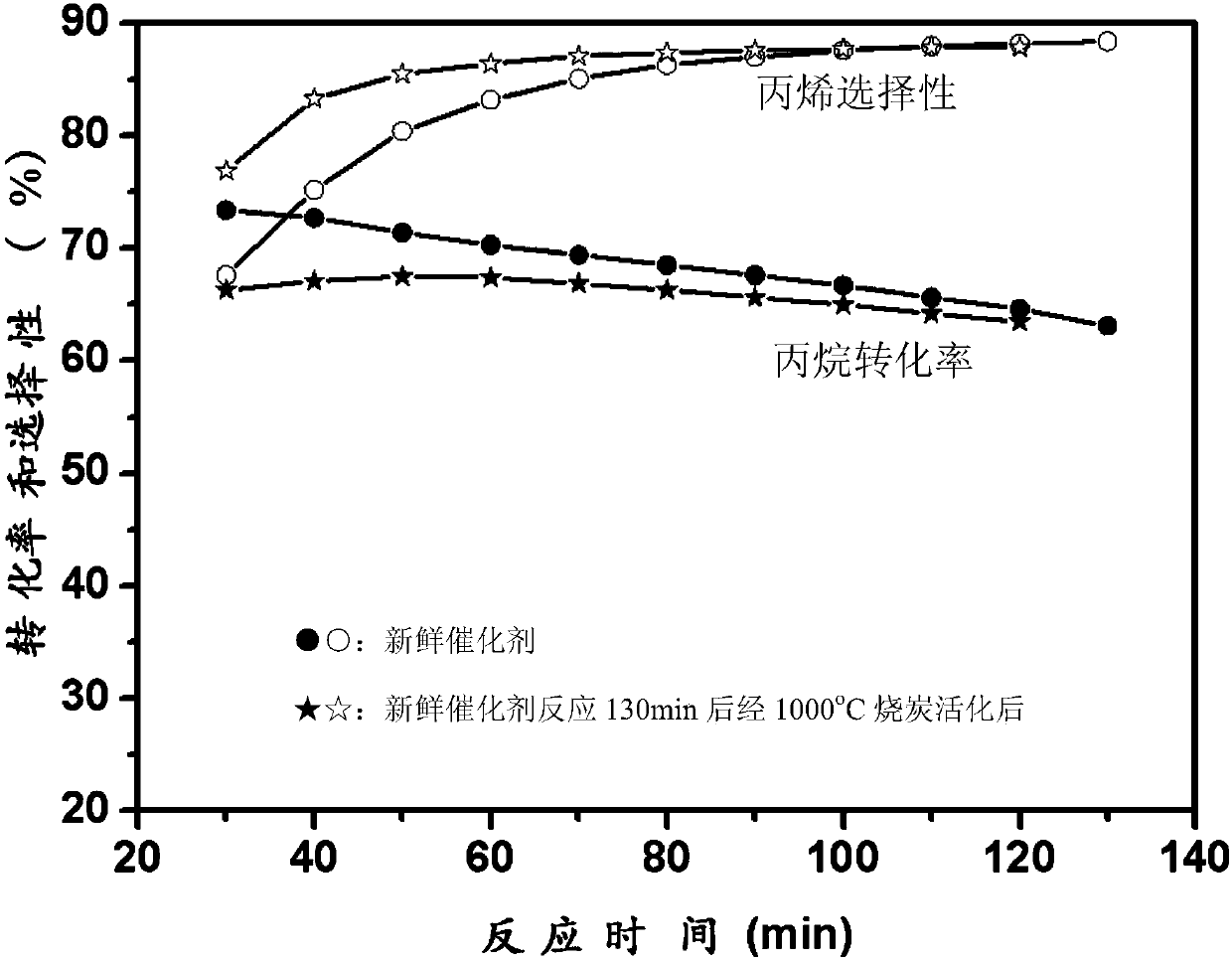
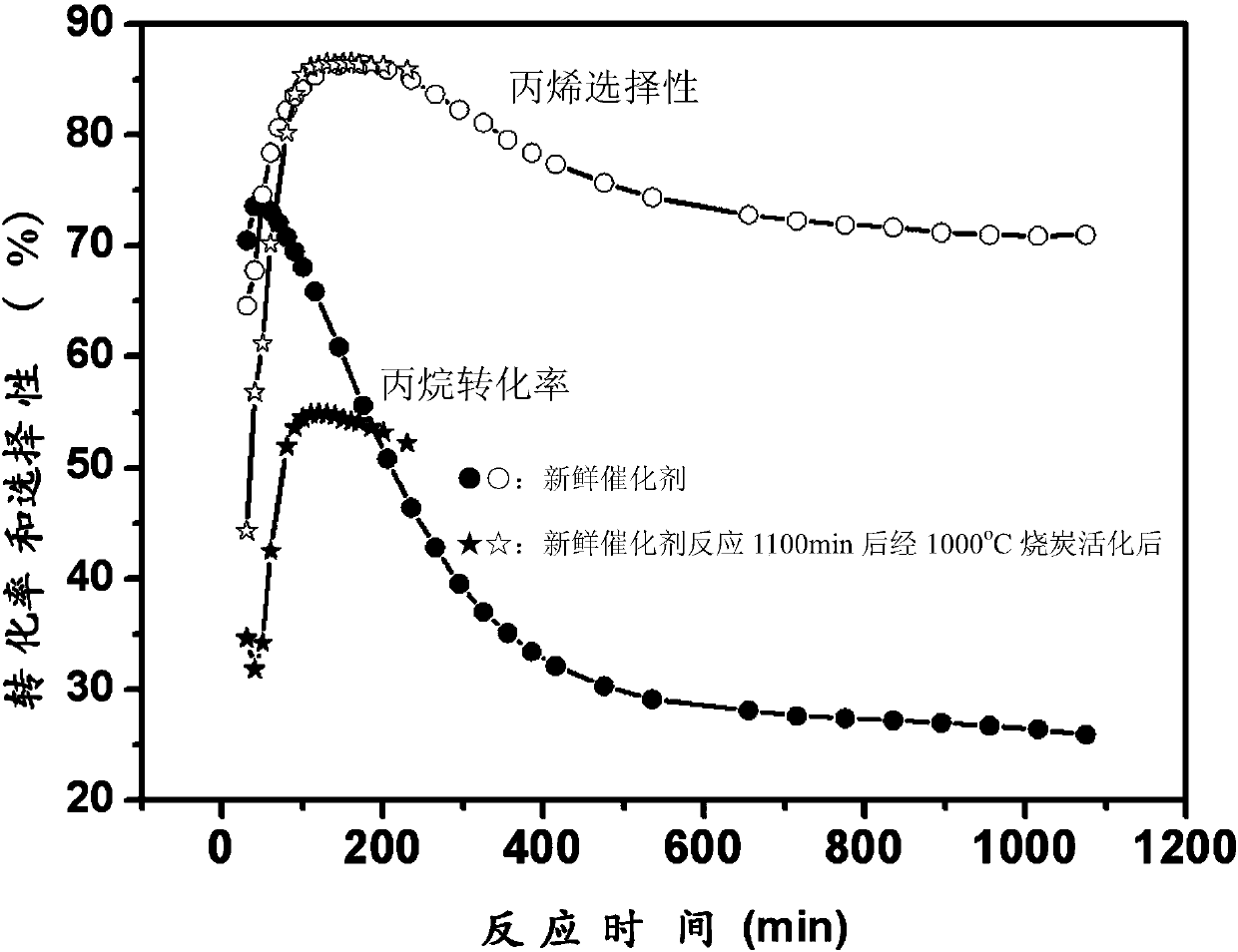
Loaded catalyst for propane dehydrogenation to propylene and preparation method thereof
ActiveCN110152650AHigh catalytic activityExcellent resistance to phase changeHeterogenous catalyst chemical elementsCatalyst activation/preparationAlkaneDehydrogenation
The invention aims to provide a loaded catalyst for propane dehydrogenation to propylene and a preparation method thereof. The catalyst consists of a Cr2O3 active component and a carrier material withhigh temperature sintering resistance, phase change resistance and high specific surface at high temperature. The chemical formula of the catalyst is yCr2O3 / MaOb.xAl2O3, y=10-30%, a=1-3, b=1-4; x=4-8, and the carrier material is metal doped aluminum oxide salt MaOb.xAl2O3. Precipitation technique, complexing technique, sol-gel technique or reverse micro-emulsion technique is employed to prepare the carrier material MaOb.xAl2O3 with high temperature sintering resistance, phase change resistance and high specific surface; impregnation process and solid phase ball mill mixing technique are adopted to prepare the yCr2O3 / MaOb.xAl2O3 loaded catalyst, y=10-30%, and the Cr2O3 active component precursor material adopts chromium nitrate, chromium acetate, chromium citrate, chromium acetylacetonateand other chromium-containing precursors. The synthesized loaded catalyst shows good catalytic activity in the reaction of direct dehydrogenation of alkane to prepare propylene, especially shows excellent sintering and phase change resistance, thus greatly improving the stability and service life of the catalyst.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI +1
Process and composition for treating metal surfaces using trivalent chromium compounds
An acidic, aqueous composition contains a trivalent chromium compound, an organo-functional silane, and a compound of a group IV-B element. The composition protects metal surfaces, preferably aluminum and aluminum alloys, against corrosion and improves their paint adhesion. The trivalent chromium compound may comprise chromium fluoride and optionally others, such as chromium nitrate. The organo- functional silane is preferably an aminopropyltriethoxy silane, and the compound of a group IV-B element is preferably fluorozirconic acid. The composition can either be dried-in-place or rinsed before a further coating layer is applied. The composition may also include at least one polymer having a plurality of both carboxylic functional groups, alone or with hydroxyl groups. A process uses the aqueous composition either with or without the organo-functional silane along with a sealing step following the application of the aqueous composition; the sealing step involves applying a sealing composition, including an organo-functional silane, to the metal surface.
Owner:BULK CHEM
Preparation method of nano tungsten powder
A preparation method of nano tungsten powder comprises the following steps: (1) dissolving ammonium metatungstate, chromium nitrate and a water-soluble carbon source substance into in heated deionized water, electrically stirring to sufficiently mix raw materials, and after uniformly mixing the raw materials, performing spray drying to obtain precursor powder, wherein the weight percentage of the water-soluble carbon source substance is 10-30%, the weight percentage of the chromium nitrate is 0.5-2% and the temperature of the deionized water is 70 DEG C; and (2) putting the precursor powder obtained in the step (1) into a tubular atmosphere furnace, performing auxiliary hydrogen reduction, and before discharging the powder out of a furnace, passivating the powder with an inert gas, wherein the temperature is 710-850 DEG C, the heating rate is 10-15 DEG C / min and the reaction time is 2-5h. The particle size of the prepared tungsten carbide is 40-70nm; after the prepared tungsten carbide is crushed, agglomeration-free nano tungsten powder can be obtained; the environment is polluted; and the development of a nanocrystalline WC-Co hard alloy can be effectively promoted.
Owner:NANCHANG UNIV
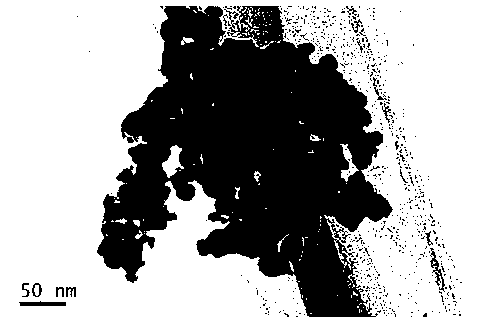
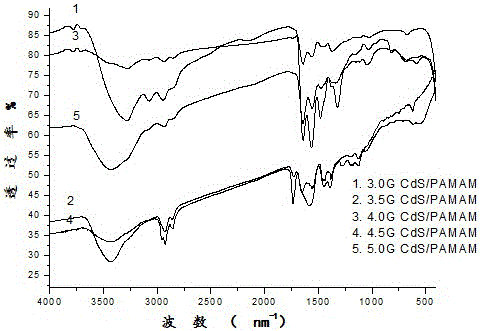
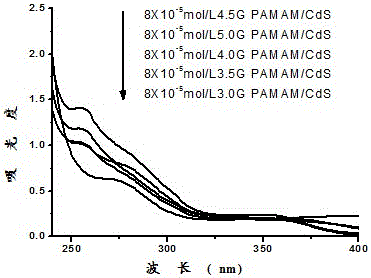
Preparation and application of CdS-PAMAM nanocomposite in detecting Cu2+
The invention discloses preparation and application of a CdS-PAMAM nanocomposite in detecting Cu2+, and belongs to the technical field of composites and ion detection.A polyamidoamine (PAMAM) dendrimer is used as a template, CdS is used a light emitting center (chromic nitrate and sodium sulfide are ion sources for synthesizing CdS), and the molecule CdS-PAMAM nanocomposite of a tree structure is synthesized in situ.According to selective testing of the CdS-PAMAM nanocomposite for metal ions, the result proves that the only the addition of copper ions has the remarkable effect on the fluorescence intensity of the composite (fluorescence of the composite is quenched and reduced from 1678 to 957), and the addition of other metal ions will not greatly change fluorescence of the composite.Accordingly, the CdS-PAMAM nanocomposite can be used for copper ions in the specific selectivity fluorescence detection environment.
Owner:LANZHOU UNIV OF ARTS & SCI
Kitchen ventilator cleaning agent capable of highly-efficiently decontaminating
InactiveCN102952649AQuick breakdownGood removal effectSurface-active detergent compositionsDetergent compounding agentsHexamethylenetetraminePolyvinyl alcohol
The invention relates to a cleaning agent, especially to a kitchen ventilator cleaning agent capable of highly-efficiently decontaminating. The agent is prepared by the following raw materials by weight parts: polyvinyl alcohol, ethylene glycol monomethyl ether, sodium dodecyl benzene sulfonate, urotropine, chromium nitrate, polyethylene glycol, lemon flavor and water with a ratio of 5 to 15 : 10 to 20 : 10 to 30 : 0.5 to 1.5 : 0.03 to 0.1 : 0.01 to 0.08 : 0.01 to 0.1 : 150 to 220. The kitchen ventilator cleaning agent capable of highly-efficiently decontaminating is environmentally-friendly, non-toxic, tasteless, non-stimulating, non-flammable and harmless to the human body and skin, does not damage galvanized coating and paint on surfaces of electric appliances, and can rapidly decompose difficult-to-remove grease and dirt attached to the surfaces of the electric appliances without dismounting the kitchen ventilator; and thus the stubborn oil stain can be easily removes and an effect of shininess as new can be achieved.
Owner:李楠
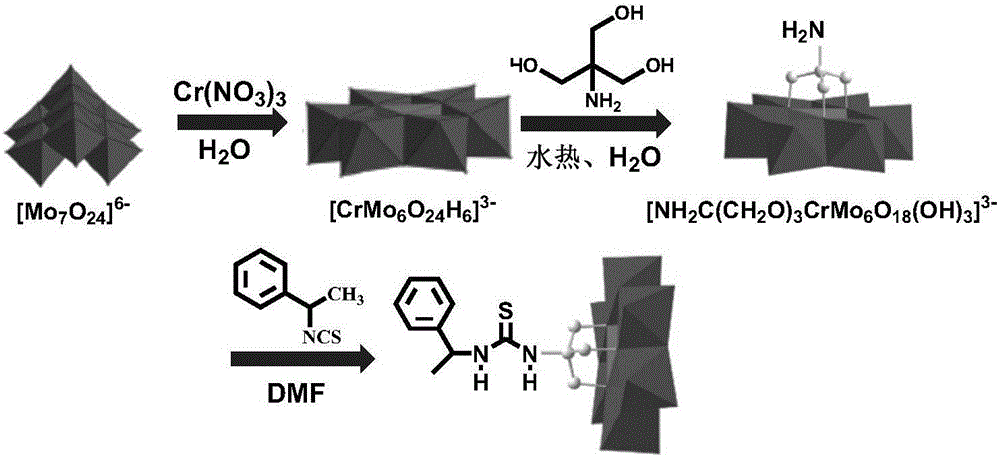
1-Phenethylthiourea modified Cr-Anderson heteropolyacid catalyst, and preparation method and application thereof
ActiveCN105833911AHigh catalytic activityReduce dosageOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAlkeneChromium nitrate
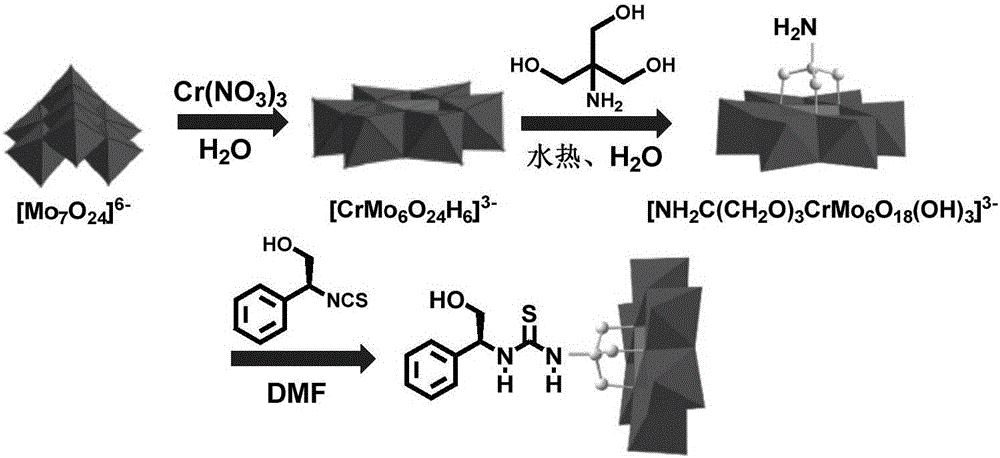
The invention discloses a 1-phenethylthiourea modified Cr-Anderson heteropolyacid catalyst, and a preparation method and an application thereof The preparation method comprises the following steps: reacting ammonium molybdate with chromium nitrate to generate a Cr-Anderson heteropolyacid (NH4)3[Cr(OH)6Mo6O18], carrying out a hydrothermal reaction on the Cr-Anderson heteropolyacid (NH4)3[Cr(OH)6Mo6O18] and trihydroxyaminomethane in a hydrothermal kettle to obtain organic one-sided amino group-modified polyoxometallate; synthesizing 1-phenethylisothiocyanic acid; and reacting the organic one-sided amino group-modified polyoxometallate with 1-phenethylisothiocyanic acid to obtain the 1-phenethylthiourea modified Cr-Anderson heteropolyacid catalyst. The preparation method has the advantages of simplicity and environmental protection. The catalyst obtained in the invention can be used in asymmetric dihydroxylation reactions of olefins, and has the advantages of high catalysis activity, recycling realization, and suitableness for industrial production.
Owner:上海元革新材料科技有限公司
Aqueous solution of chromium salt and method for producing same
Disclosed is an aqueous solution of a chromium salt which is characterized in that the oxalic acid content is not more than 8 weight% relative to chromium. In this aqueous solution of a chromium salt, the total organic carbon is not more than 4 weight% relative to chromium. The chromium salt may preferably be a chromium chloride, a chromium phosphate or a chromium nitrate. The chromium chloride may preferably contain a basic chromium chloride represented by the following composition formula: Cr(OH)xCly (wherein 0 < x <= 2, 1 <= y < 3, and x + y = 3). The chromium phosphate may preferably be one represented by the following composition formula: Cr(H3-3 / nPO4)n (wherein n is a number satisfying 2 <= n <= 3). The chromium nitrate may preferably be a basic chromium nitrate represented by the following composition formula: Cr(OH)x(NO3)y (wherein 0 < x <= 2, 1 <= y < 3, and x + y = 3).
Owner:NIPPON CHECMICAL IND CO LTD
Catalyst and preparation method thereof
InactiveCN102091639AEfficient removalWater contaminantsMetal/metal-oxides/metal-hydroxide catalystsCobaltChromium nitrate
The invention relates to a catalyst and a preparation method thereof. The preparation method comprises the following steps of: weighing 1kg of carrier active carbon; removing impurities and dust in clear water; weighing 70g of ferric nitrate, 60g of nickel nitrate, 30g of manganous nitrate, 10g of potassium nitrate, 30g of sodium metavanadate, 30g of cobalt nitrate, 60g of cerous nitrate, 80g of ammonium molybdate, 20g of chromium nitrate, 0.5g of platinum and 0.5g of aurum and mixing and dissolving; then charging the cleaned active carbon into a prepared solution and soaking for 48 hours; fully adsorbing metal salt by the active carbon; after completely soaking, drying in the sun; after drying in the sun, charging the metal salt into an oven for curing at high temperature; after curing, discharging and naturally cooling; and then screening and packaging a finished product for storage. The invention has the advantage of effectively removing heavy metal ions and organic matter enriched in sewage treatment.
Owner:浙江亿康环保工程有限公司
Preparation method of CeO2-Cr2O3-Nb2O5 compound oxide catalyst
InactiveCN105251476ALow costHigh activityDispersed particle separationMetal/metal-oxides/metal-hydroxide catalystsChlorobenzeneNiobium
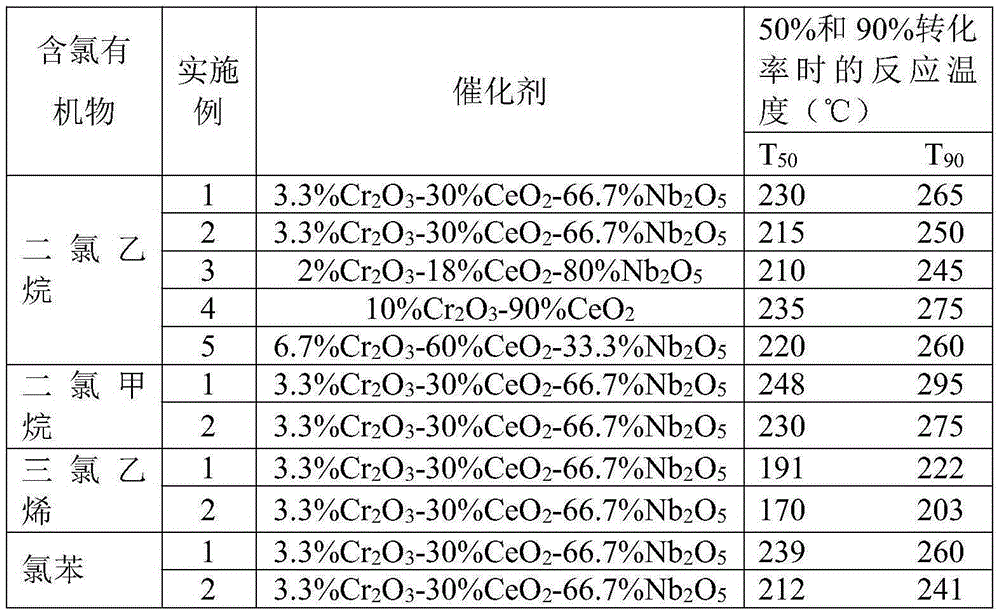
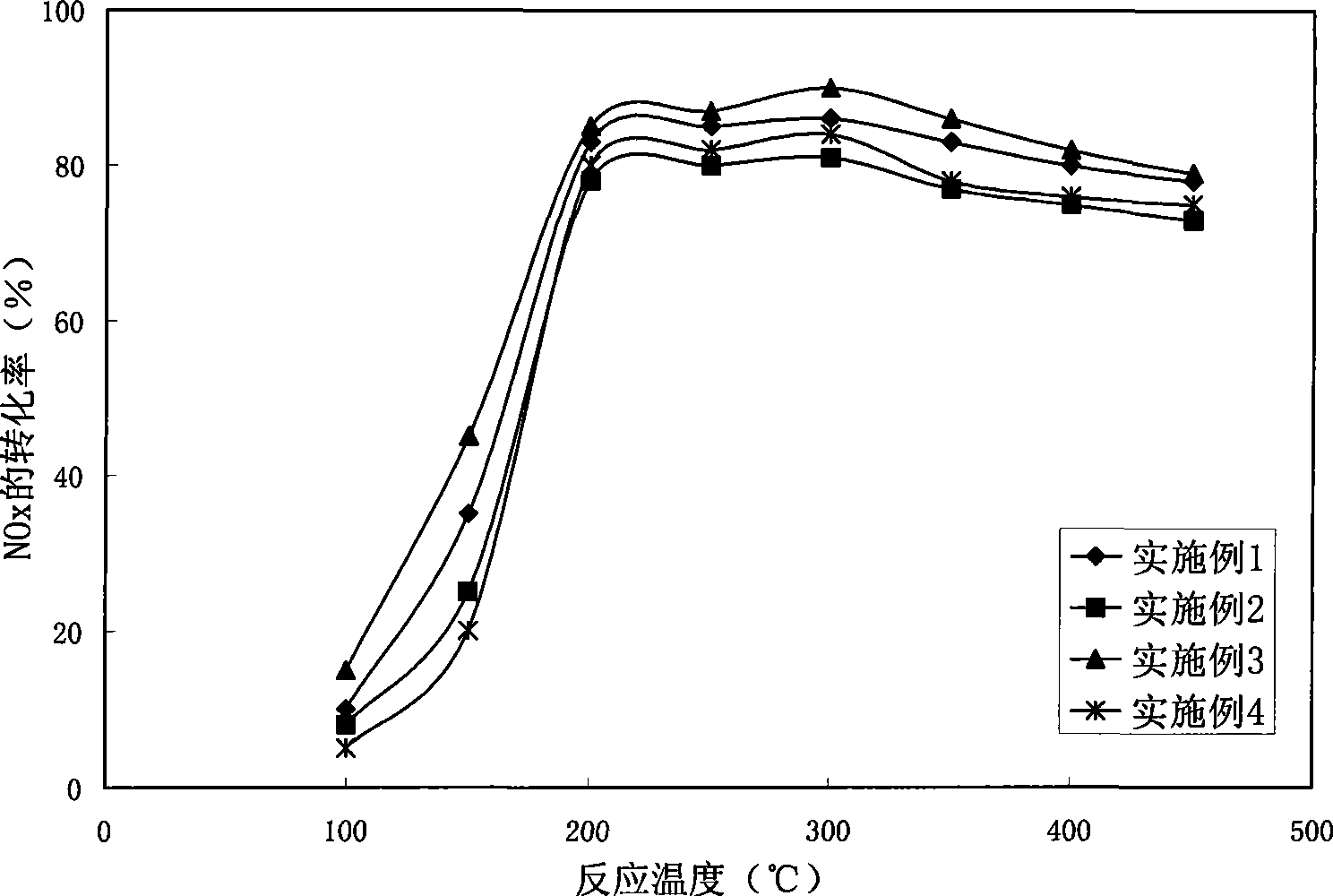
The invention provides a preparation method of a CeO2-Cr2O3-Nb2O5 compound oxide catalyst. Chromium nitrate, cerous nitrate and acidic Nb2O5 oxide or niobium oxalate ammonia serve as raw materials, the CeO2-Cr2O3-Nb2O5 compound oxide catalyst is prepared through a precipitation-deposition or co-precipitation method, the molecular ratio of Ce to Cr in the catalyst is 4:1, and the mass ratio of (Cr2O3 + CeO2) to Nb2O5 is 4:1-1:9. The catalyst is simple in preparation technology and low in cost and has good catalytic degradation activity and stability on dichloromethane (DCM), ethylene dichloride (DCE), trichloroethylene (TCE), chlorobenzene and other chlorine-containing organic compounds, CO2 and HCl are high in selectivity, and no polychlorocarbon or other by-products are generated.
Owner:ZHEJIANG UNIV
Perovskite substance, thermal power plant denitration compound catalyst and preparation methods thereof
InactiveCN106140145ASolve ammonia slip problemEasy to operateGas treatmentHeterogenous catalyst chemical elementsPhosphateNitration
The invention discloses a perovskite substance, a thermal power plant denitration compound catalyst and preparation methods thereof and belongs to the technical field of flue gas denitration catalysts. In order to solve the problem that existing perovskite substance preparation processes are complex, the invention provides the preparation method of the perovskite substance. The preparation method of the perovskite substance includes: dissolving lanthanum nitrate, strontium nitrate and chromium nitrate as raw material precursors in water; calcining at high temperature to obtain the perovskite substance. In order to solve the problem that existing denitration catalysts cause ammonia escape during nitration, the invention further provides the preparation method of the thermal power plant denitration compound catalyst. The preparation method of the thermal power plant denitration compound catalyst includes following steps: kneading titanium dioxide mixture, active liquid, water, glycerin, ethylene glycol, glass fiber and aluminum dihydrogen phosphate to obtain ceramic pug; performing vacuum extrusion to obtain a cellular ceramic blank; subjecting the cellular ceramic blank to steam drying and calcining to obtain the thermal power plant denitration compound catalyst. The catalyst is high in nitration efficiency, and the problem of ammonia escape is solved.
Owner:无锡市华东电力设备有限公司
Method for preparing nitrous oxides selectivity reduction catalyst on metal alloy carrier
InactiveCN101249444ALow efficiencyIncrease resistance to poisoningDispersed particle separationMetal/metal-oxides/metal-hydroxide catalystsCerium nitrateDip-coating
The invention belongs to a field of diesel-engined car tail gas cleaning and relates to a method for preparing a catalyst with optimized performance on metal alloy support for selectively reducing nitrogen oxide. The method includes preparing nickel nitrate solution, manganese nitrate solution, copper nitrate solution, cobalt nitrate solution, ferric nitrate solution, cerium nitrate solution and chromium nitrate solution with the distilled water as solvent; selecting 2-4 kinds of solutions from the solutions and mixing with silver nitrate solution or palladium nitrate of equivalent volume to prepare catalyst solution; dip-coating the catalyst solution on a glass ceramic coating of metal alloy support by; air-drying the coated metal carrier at room temperature; drying at 120-180 DEG C; repeating the coating and the drying for 5-8 times; and calcining at 600-1,000 DEG C for 1-5h to activate and mold. The method has the advantages of low cost, good initiation and catalytic characteristic effect.
Owner:CHANGCHUN JLU KENUO TECH CO LTD
Method for preparing catalytic material used in hydrogen production through catalytic photolysis of water by visible light
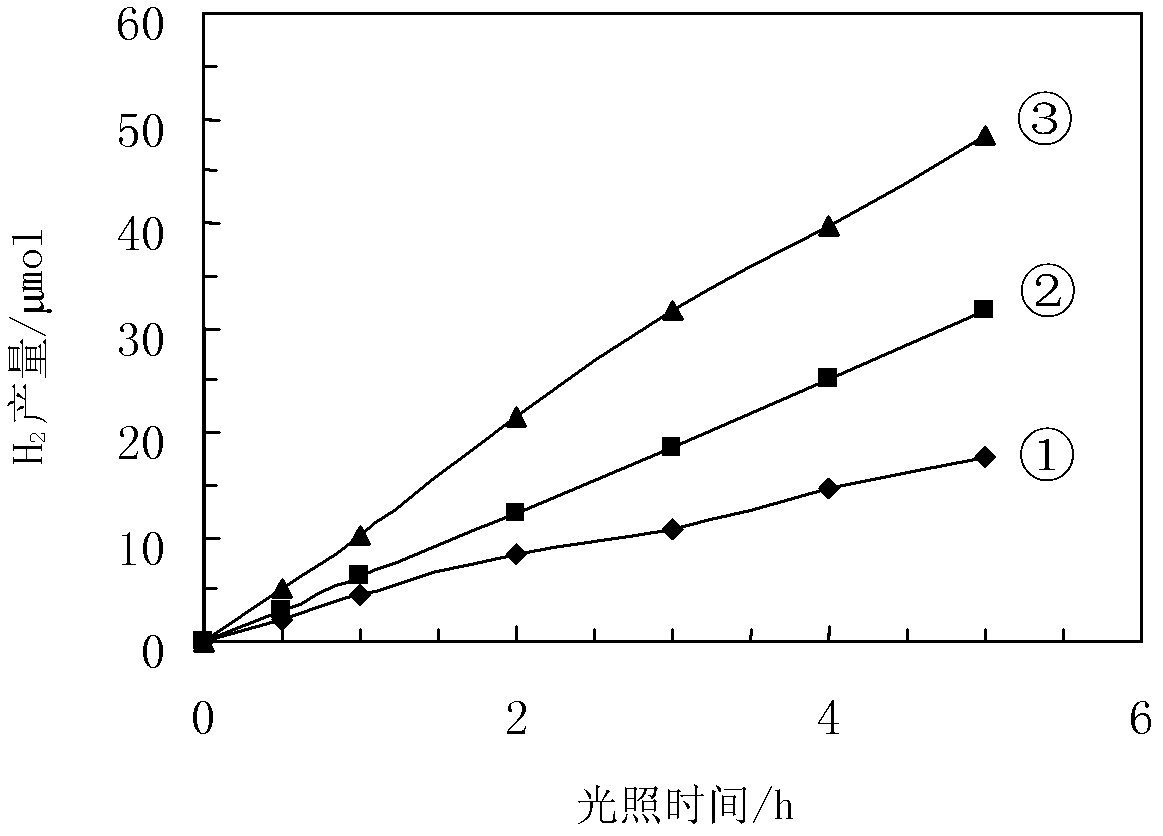
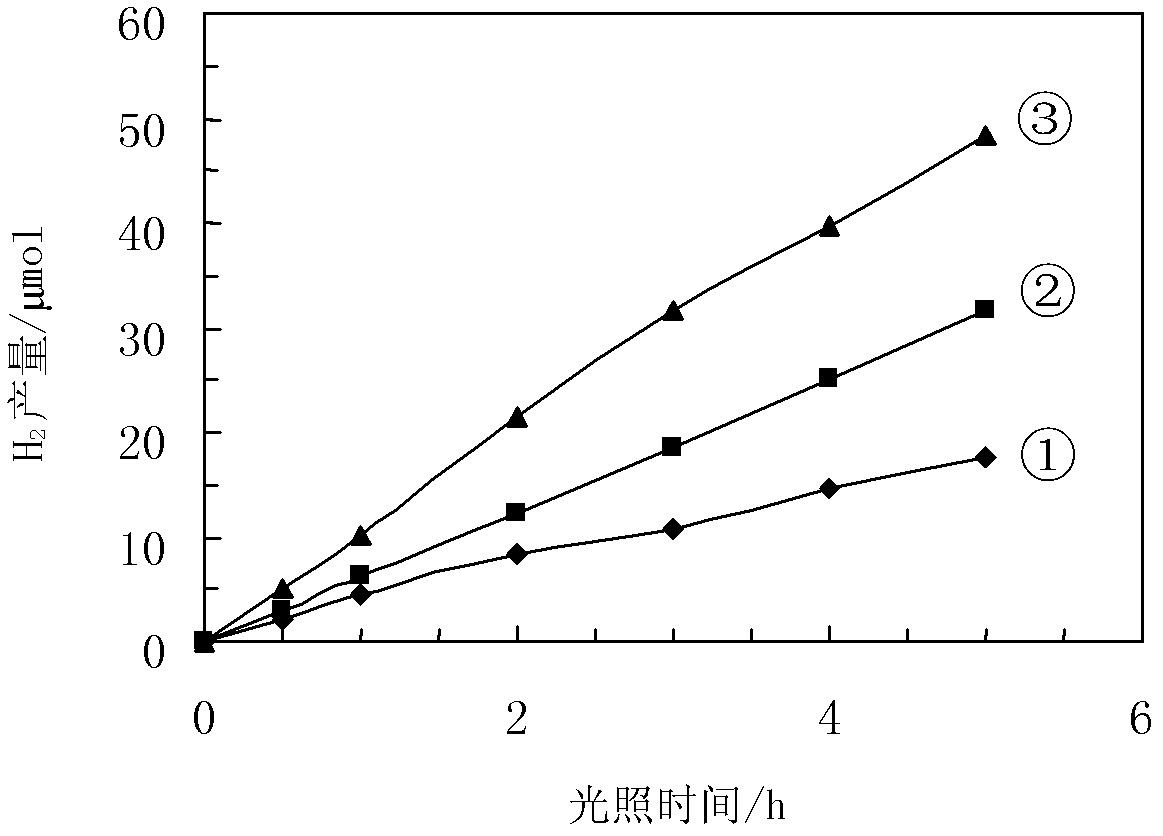
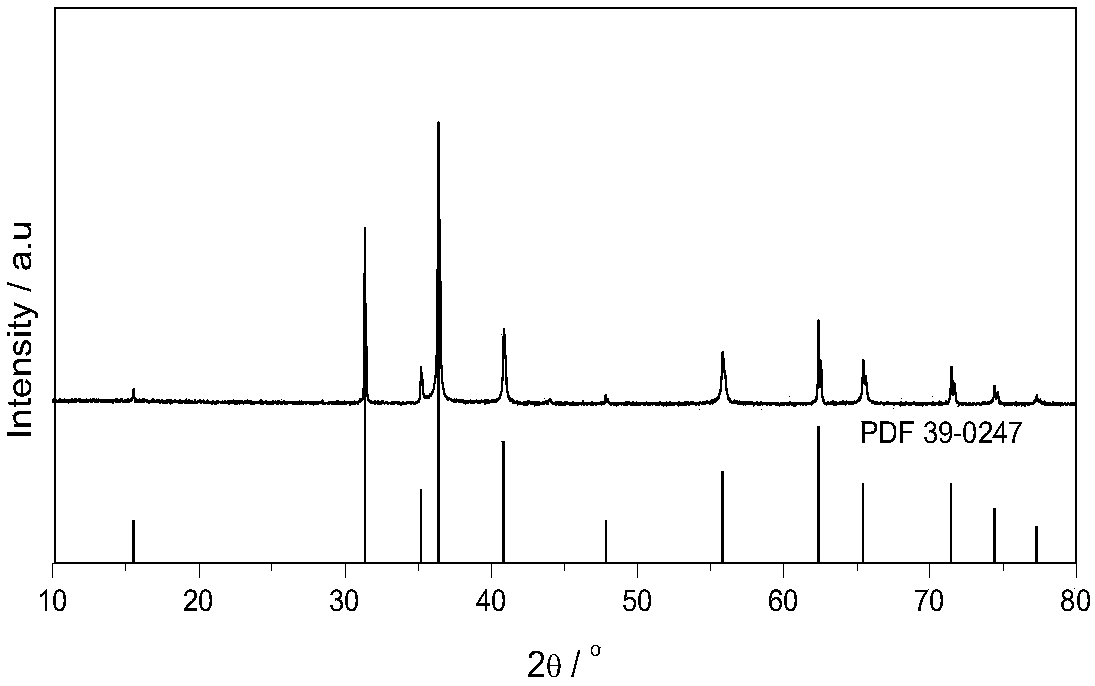
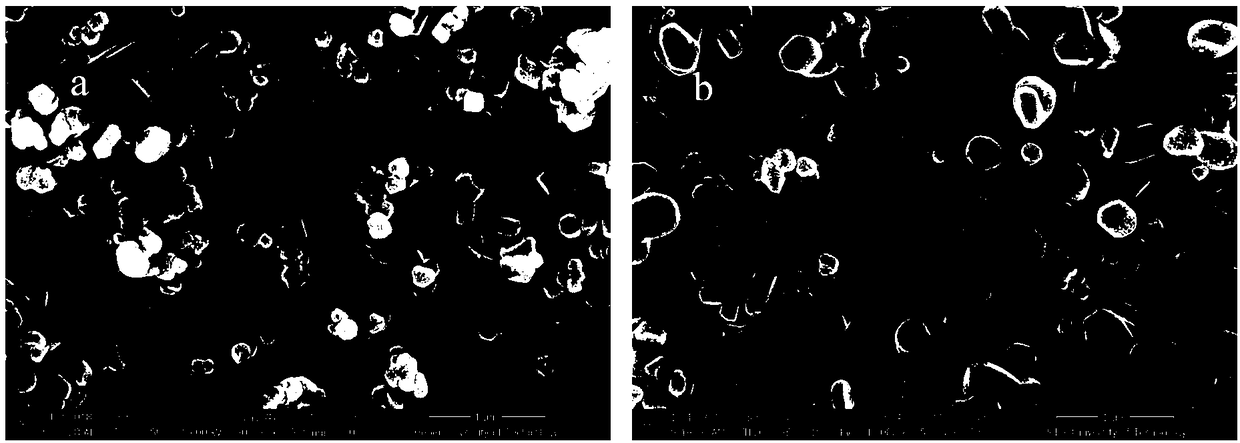
InactiveCN102407103ATake advantage ofImprove photocatalytic activityEnergy inputHydrogen productionChromium nitratePhotochemistry
The invention relates to a method for preparing a catalytic material used in hydrogen production through catalytic photolysis of water by visible light. The method is mainly characterized in that a compound oxide BaCr2O4 is prepared from barium nitrate and chromium nitrate serving as main raw materials by a sol-gel method in the presence of EDTA (ethylene diamine tetraacetic acid), citric acid, tartaric acid and the like serving as a compound complexant. The method has the following steps of: drying and crushing the compound oxide BaCr2O4, soaking in a cerous nitrate solution with a certain concentration, magnetically stirring for 3 hours, and standing; and heating and evaporating to dryness, drying for 10 hours at the temperature of 120 DEG C, roasting for 10 hours at the temperature of 350 DEG C, and roasting for 12 hours at the temperature of 1200 DEG C to obtain a doped Ce / BaCr2O4 photo-catalytic material. Compared with a RuO2 / TiO2 nano crystal photochemical catalyst, the photo-catalytic material is available in raw materials, low in cost, high in catalytic hydrogen production efficiency, has visible light catalytic activity, and is easy to apply and prompt, and can fully transform and use solar energy.
Owner:XIAN UNIV OF TECH
Sol-gel preparation methd of CuCrO2 powder
ActiveCN108187686AEvenly dispersedUniform decompositionCatalyst activation/preparationMetal/metal-oxides/metal-hydroxide catalystsWater bathsDispersity
The invention discloses a sol-gel preparation methd of CuCrO2 powder. The method comprises the following steps: preparing a mixed solution from copper nitrate and chromium nitrate with equal mole number; adding citric acid and ethylene glycol into the mixed solution, and stirring to form a colorless transparent solution; performing water-bath heating at 75-90 DEG C for 4-6h to form thick liquid; cooling to room temperature, adding an aqueous solution of ammonia into the thick liquid, transferring to water-bath at 75-90 DEG C for heating to obtain a thick matter; performing vacuum drying for 18-40h to obtain gray powder; transferring the gray powder into a tubular furnace, and roasting at 300-550 DEG C for 0.5-1.5h; cooling and grinding; roasting in a nitrogen atmosphere at 800-950 DEG C for 1-3h to obtain CuCrO2 powder. The CuCrO2 powder prepared by the method has the advantages of fine particles, high dispersity, high crystal integrity and relatively uniform particle size distribution.
Owner:HEBEI UNIV OF TECH
Metal organic skeleton-graphite oxide nano composite adsorption material and preparing method thereof
ActiveCN102335592BLarge specific surface areaHigh porosityOther chemical processesMetal-organic frameworkChromium nitrate
The invention discloses a metal organic skeleton-graphite oxide nano composite adsorption material and a preparing method thereof. The material is composed of graphite oxide and chromium-based metal organic skeleton. The preparing method of the material comprises the following steps of: dissolving chromium nitrate and terephthalic acid in deionized water, stirring and simultaneously adding hydrofluoric acid dropwise, then adding graphite oxide and uniformly stirring to obtain reaction liquid, and conducting hydro-thermal reaction under program temperature controlling; and after sequentially conducting washing with water and washing with ethanol for the decomposed materials, centrifuging, drying and obtaining the metal organic skeleton-graphite oxide nano composite adsorption material. Theadsorption capacity of the adsorption material for hydrocarbon volatile organic matters is greatly increased, and the adsorption material is easy for desorption and regeneration. The preparing methodhas simple process and low cost.
Owner:SOUTH CHINA UNIV OF TECH
(S)-1-(2-hydrox-1-phenethyl)thiourea modified Cr-Anderson heteropolyacid catalyst, and preparation method and application thereof
ActiveCN105833909AHigh catalytic activityHigh enantioselectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsThioureaIsothiocyanic acid
The invention discloses an (S)-1-(2-hydrox-1-phenethyl)thiourea modified Cr-Anderson heteropolyacid catalyst, and a preparation method and an application thereof The preparation method comprises the following steps: reacting ammonium molybdate with chromium nitrate to generate a Cr-Anderson heteropolyacid (NH4)3[Cr(OH)6Mo6O18], carrying out a hydrothermal reaction on the Cr-Anderson heteropolyacid (NH4)3[Cr(OH)6Mo6O18] and trihydroxyaminomethane to obtain organic one-sided amino group-modified polyoxometallate; and synthesizing (S)-1-(2-hydrox-1-phenethyl)isothiocyanic acid from L-phenylglycinol, and reacting the (S)-1-(2-hydrox-1-phenethyl)isothiocyanic acid with the organic one-sided amino group-modified polyoxometallate to obtain the target catalyst. The preparation method has the advantages of simplicity, mild reaction conditions and environmental protection. The heteropolyacid catalyst obtained in the invention can be used in asymmetric dihydroxylation reactions of olefins, and has the advantages of high catalysis activity, high enanioselectivity, recycling realization, and suitableness for industrial production.
Owner:上海元革新材料科技有限公司
Process for producing tungsten carbide cobalt chrome metal composite powder
The invention discloses a process for producing tungsten carbide cobalt chrome metal composite powder, comprising the following production steps: pre-preparing ammonium tungstate solution, cobalt nitrate solution and chromium nitrate solution; controlling the pH value by heating after controlling the concentration of ammonia in the ammonium tungstate solution; respectively adding the cobalt nitrate solution and chromium nitrate solution, and then stopping heating; adopting vacuum filtration to the solution for solid-liquid separation after cooling; obtaining a coprecipitation crystal; using ammonium nitrate solution to wash and soak the crystal and then draining and baking the crystal to implement calcination; using hydrogen to reduce the calcinated metal oxide to be metal reduction powder; and then using carbon dioxide gas to lead the metal reduction powder to be insulated from the air; adding carbon to the metal reduction powder for ball milling after low-temperature cooling; implementing low-temperature carbonization to the metal reduction powder which is added with the carbon; and finally obtaining the finished product by grinding, crushing and sieving. The process for producing tungsten carbide cobalt chrome metal composite powder has high product purity, high combined carbon content, good uniformity, normal distribution, complete particle size development, good main chemical component uniformity, and excellent use performance.
Owner:GUANGDONG XIANGLU TUNGSTEN
Method for preparing hydrophobic Cr-Ce-based catalyst through ultrasound-hydrogen reduction
ActiveCN102350338AHigh activityHydrophobicDispersed particle separationMetal/metal-oxides/metal-hydroxide catalystsCerium nitratePtru catalyst
The invention discloses a method for preparing a hydrophobic Cr-Ce-based catalyst through ultrasound-hydrogen reduction. The method comprises the following steps: 1, codissolving chromium nitrate and cerium nitrate in deionized water to prepare a mixed solution of chromium nitrate and cerium nitrate; 2, adding Al2O3 particles to the mixed solution, oscillating and carrying out ultrasonic dipping at a constant temperature, drying, and calcinating to obtain solid particles; and 3, carrying out reduction processing on the solid particles in hydrogen, and calcinating the solid particles in air toprepare the hydrophobic Cr-Ce-based catalyst. The catalyst prepared through the method of the invention is mainly used for catalyzing the combustion of volatile organic compounds containing chlorine,the method which allows noble metals to be replaced with transition metals allows the cost to be reduced and the high activity of the catalyst to be simultaneously maintained, and the catalyst can catalyze the combustion of the volatile organic compounds containing chlorine at a low temperature; and the catalyst which still has a high activity at a high extraneous humidity has a good practical application prospect.
Owner:SOUTH CHINA UNIV OF TECH
Method for regenerating activity of deactivated ferritic catalyst
ActiveCN102000613AIdeal Regenerated Catalyst MethodLittle effect on activityCatalyst regeneration/reactivationChemical recyclingIndiumChromium nitrate
The invention provides a method for regenerating activity of a deactivated ferritic catalyst. The ferritic catalyst is a catalyst for catalyzing 2,6-xylenol to produce polyphenylene oxide and comprises Fe2O3 and In2O3. The method comprises the following steps of: introducing air into a deactivated catalyst; sintering the catalyst at the temperature of between 450 and 500 DEG C to remove coked carbon in the catalyst; crushing; thermally dissolving the crushed catalyst with 10 to 63 percent of nitric acid or a mixed liquor of the nitric acid and hydrochloric acid at the temperature of between 60 and 150 DEG C; detecting the acidulated reactive fluid; calculating according to detection results; and correspondingly adding required chromium nitrate, sodium silicate and indium.
Owner:NANTONG XINGCHEN SYNTHETIC MATERIAL
Passivation technology of aluminum alloy cylinder cover
InactiveCN104250755AReduce pollutionLow costMetallic material coating processesChromium nitrateDodecylsulfonic acid
The invention discloses a passivation technology of an aluminum alloy cylinder cover. A passivation liquid used by the passivation technology comprises 4-6g / L of sodium silicate, 13-15g / L of titanium dioxide, 20-23g / L of nitric acid having a concentration of 68%, 15-17g / L of potassium permanganate, 3-5g / L of sodium peroxide, 6-8g / L of ammonium bifluoride, 22-24g / L of ferrous chloride, 5-9g / L of chromium nitrate, 3-5g / L of zirconium nitrate, 14-16g / L of sodium dodecylsulfate and the balance water. The passivation technology comprises the following steps of 1, carrying out alkali washing and carrying out cleaning by clear water, 2, carrying out pickling and carrying out cleaning by clear water, 3, preparing the passivation liquid from the above components, putting the passivation liquid into a passivation tank, diluting the passivation liquid by 4-6 times, controlling a temperature of the passivation liquid in a range of 35-45 DEG C and a pH value in a range of 3-5, putting a supersonic generator at the bottom of the passivation tank, carrying out passivation for 13-17min, then carrying out cleaning by water and carrying out natural drying.
Owner:无锡杨市表面处理科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com