Method for preparing catalytic material used in hydrogen production through catalytic photolysis of water by visible light
A technology of photocatalytic water splitting for hydrogen production and catalytic materials, applied in chemical instruments and methods, hydrogen production, physical/chemical process catalysts, etc. , to solve the problem of low utilization of visible light, conducive to popularization and application, and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
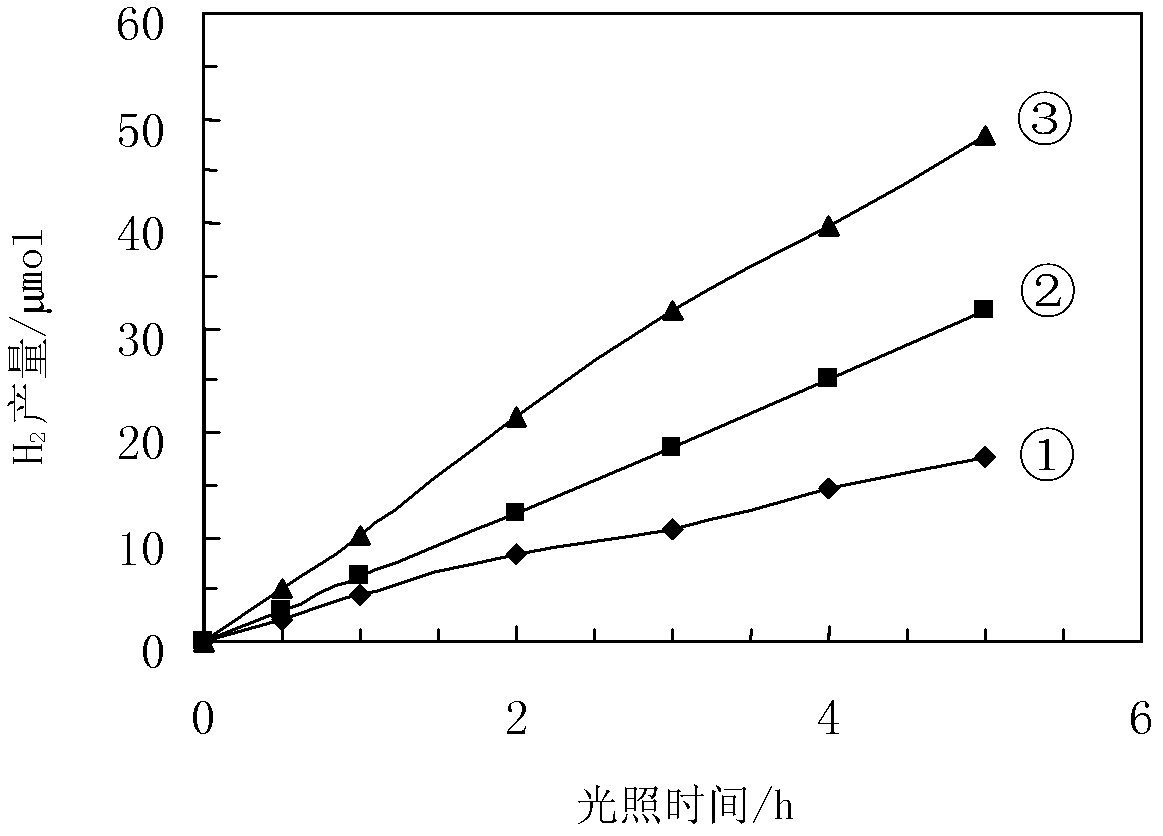
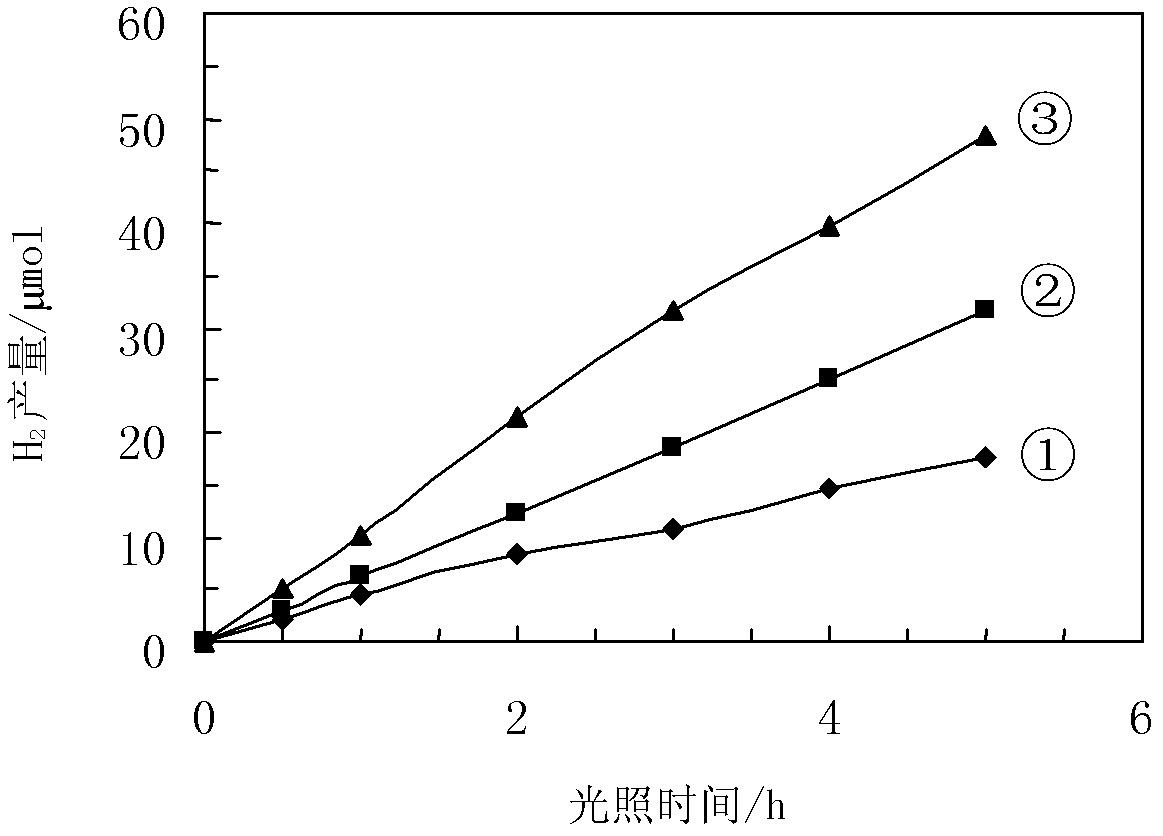
Image
Examples
preparation example Construction
[0020] The invention provides a preparation method of a catalytic material for hydrogen production by photocatalytic photolysis of water with visible light, which is specifically implemented according to the following steps:
[0021] step 1,
[0022] First, dissolve EDTA in ammonia water to make a 2mol / L EDTA solution;
[0023] Step 2,
[0024] Ba(NO 3 ) 2 and Cr(NO 3 ) 3 9H 2 O is dissolved in water to make a mixed solution, then add the EDTA solution configured in step 1 to the mixed solution, keep the amount of the EDTA substance the same as the amount of the total metal ion substance, then add the substance with the nitrate Auxiliary complexing agent with the same amount, the auxiliary complexing agent is one of citric acid, tartaric acid, oxalic acid, stir to dissolve, and then use ammonia water to adjust the pH value of the solution to 5-7;
[0025] Step 3,
[0026] Put the mixed solution prepared in step 2 into a water bath at 70-80°C, stir electrically until th...
Embodiment 1
[0032] Ba(NO 3 ) 2 1.31g and Cr(NO 3 ) 3 9H 2 Dissolve O2.38g in a beaker with deionized water, then add 7.7g of citric acid to the mixed solution, stir to dissolve it, and then add 7.5mL of 2mol / L EDTA solution. Adjust pH to pH=6 with ammonia water. Then put the mixed solution into a water bath at 80° C., and stir at constant temperature for a certain period of time. During the stirring process, the viscosity of the solution continued to increase until the system could be drawn, and the water bath heating was stopped. Cool in air to obtain a gel. It was dried at a temperature of 120° C. for 6 hours to obtain a xerogel. The dry gel is calcined at 350°C for 6 hours, and then calcined at 1000°C for 8 hours to obtain the green powder of BaCr 2 o 4 catalyst. According to Ce: BaCr 2 o 4 Weigh Ce(NO 3 ) 4 NH 4 NO 3 0.033g in a beaker, add appropriate amount of water to dissolve, then add 1.0gBaCr 2 o 4 For the powder, after ultrasonic dispersion for 15 minutes, mag...
Embodiment 2
[0034] According to the stoichiometric ratio of 1:2, Ba(NO 3 ) 2 2.62g and Cr(NO 3 ) 3 9H 2 O4.76g was dissolved in a beaker with deionized water, then 15.4g oxalic acid was added to the mixed solution, stirred to dissolve it, and then 15mL of 2mol / L EDTA solution was added. Adjust the acidity and alkalinity to pH=7 with ammonia water. Then the mixed solution was put into a water bath at 75° C. and stirred at constant temperature. During the stirring process, the viscosity of the sol continued to increase until the system could be drawn, and the water bath heating was stopped. Cool in air to obtain a gel. It was dried at 120° C. for 10 h to obtain a xerogel. The dry gel is calcined at 350°C for 10h, and then calcined at 900°C for 12h to obtain a green powder of BaCr 2 o 4 catalyst. According to Ce:BaCr 2 o 4 Weigh Ce(NO 3 ) 4 NH 4 NO 30.066g in a beaker, add appropriate amount of water to dissolve, then add 2.0gBaCr 2 o 4 For the powder, after ultrasonic disp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com