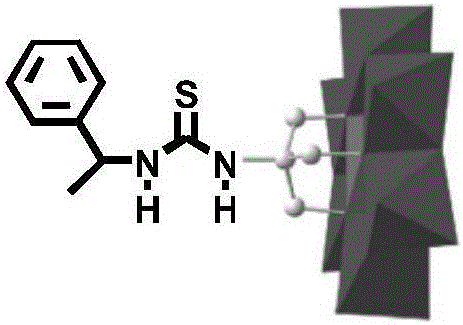
1-Phenethylthiourea modified Cr-Anderson heteropolyacid catalyst, and preparation method and application thereof
A technology of phenethyl thiourea and heteropoly acid, applied in the field of catalytic chemistry, can solve the problems of inability to explain the synergy between chiral amines and polyacids, no clear molecular structure, and inability to further explore the catalytic mechanism, etc. Reaction conditions temperature, high catalytic activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
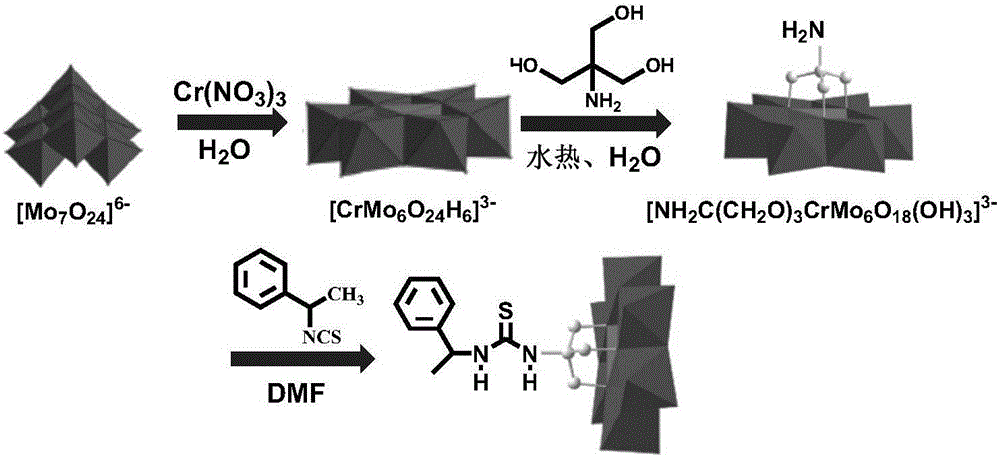
[0029] Cr-Anderson type heteropolyacid precursor (NH 4 ) 3 [Cr(OH) 6 Mo 6 o 18 ] preparation
[0030] Take 7.41g (6mmol) of ammonium molybdate and add it to 20mL of deionized water, stir well to obtain a clear liquid, then add concentrated nitric acid dropwise, and adjust the pH of the system to 4~5. Heat to boiling, maintain strong stirring, and start to slowly add 1.66 g (7 mmol) chromium nitrate solid, stir until a large amount of pink solid is produced, stop stirring, and let stand for 15-30 minutes. Suction filtration to obtain 5.26 g of pink crystalline solid material with a yield of 82%.
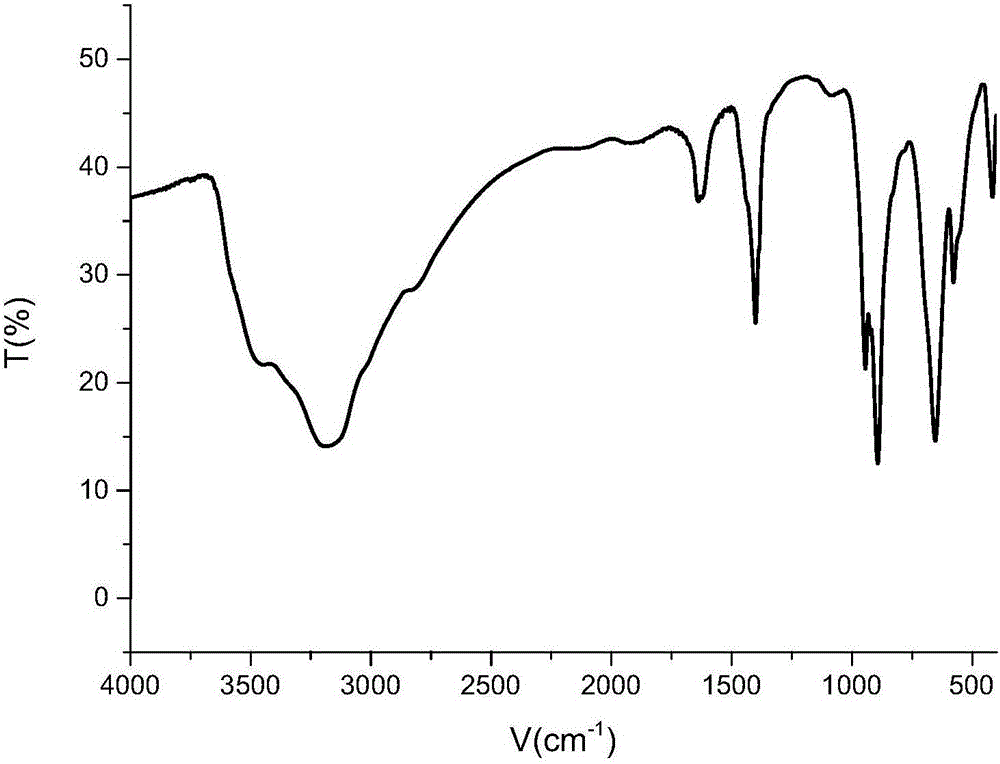
[0031] Parent (NH 4 ) 3 [Cr(OH) 6 Mo 6 o 18 ] for the infrared spectrum image 3 .
Embodiment 2
[0033] Preparation of 1-phenylethyl isothiocyanate
[0034]Add 1-phenylethylamine (0.606g, 5mmol) into a dry reaction vessel, dissolve it with 20mL of ethyl acetate, then slowly add CS 2 (0.1142g, 15mmol) and triethylamine (0.506mg, 5mmol), after stirring the reaction at room temperature for 1h, then adding di-tert-butyl dicarbonate (Boc 2 O) (1.091mg, 5mmol) and 4-dimethylaminopyridine (DMAP) (18mg, 0.15mmol), after stirring and reacting at room temperature for 2h (gas is generated during the stirring process, attention should be paid to degassing and decompression), which can be obtained 0.79 g of 1-phenylethyl isothiocyanate. Yield 97%.
[0035] The NMR spectrum of 1-phenylethyl isothiocyanate is shown in Figure 4 , the specific data are as follows:
[0036] 1 H NMR (501MHz, CDCl 3 ) δ 7.3-7.5 (dt, J=12.3, 7.7Hz, 5H), 4.90-4.95 (q, J=6.7Hz, 1H), 1.65-1.70 (d, J=6.8Hz, 3H).
Embodiment 3
[0038] Preparation of Cr-Anderson Polyoxometalates Modified with One-side Amino Group
[0039] The above-obtained Anderson matrix (NH 4 ) 3 [Cr(OH) 6 Mo 6 o 18 ] 1.071g (1mmol) was dissolved in 10mL deionized water to obtain a light red clear liquid, and then 0.402g (3mmol) of trishydroxyaminomethane was slowly added. Then add the above system into a hydrothermal kettle, heat it to 140°C, and react for 24 hours, then add 4.83g of tetrabutylammonium bromide into the above solution at 85°C, and a large amount of pink precipitate, that is, the crude product, will be produced. Filter to get a red liquid, place it to get crystals, which is the organic one-side amino modified Cr-Anderson polyacid [TBA] 3 {[NH 2 C(CH 2 O) 3 ]CrMo 6 o 18 (OH) 3}.
[0040] The infrared spectrum of the Cr-Anderson type polyoxometalate modified with one side amino group is as follows: Figure 5 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com