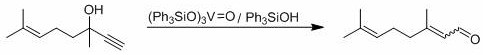Method for preparing citral through dehydrolinalool rearrangement reaction
A technology for dehydrolinalool and rearrangement reactions, applied in catalytic reactions, preparation of carbon-based compounds, chemical instruments and methods, etc., to achieve high reactivity and selectivity, convenient source, good economic benefits and application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
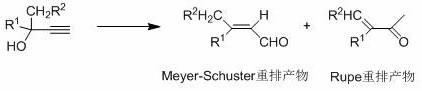
Method used
Image
Examples
Embodiment 1
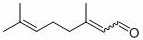
[0035] 0.152g (1mmol) dehydrolinalool, 3.26mg (0.01mmol) MoO 2 (acac) 2 , 55.6mg (0.2mmol) Ph 3 Add PO, 35.6mg (0.2mmol) 4-tert-butylbenzoic acid and 4mL solvent toluene into a thick-walled pressure-resistant reaction bottle, and put it into a 120 o After reacting 7h in C oil bath, monitor reaction by thin-layer chromatography completely, by 1 H-NMR internal standard method was used to measure the yield of the target product, and the yield of the target product citral was measured to be 53%.
Embodiment 2
[0037] With 0.152g (1mmol) dehydrolinalool, 4.89mg (0.015mmol) MoO 2 (acac) 2 , 55.6mg (0.2mmol) Ph 3 Add PO, 35.6mg (0.2mmol) 4-tert-butylbenzoic acid and 4mL solvent toluene into a thick-walled pressure-resistant reaction bottle, and put it into a 120 o After reacting 7h in C oil bath, monitor reaction by thin-layer chromatography completely, by 1 H-NMR internal standard method was used to determine the yield of the target product, and the yield of the target product citral was measured to be 67%.
Embodiment 3
[0039] With 0.152g (1mmol) dehydrolinalool, 9.78mg (0.03mmol) MoO 2 (acac) 2 , 55.6mg (0.2mmol) Ph 3 Add PO, 35.6mg (0.2mmol) 4-tert-butylbenzoic acid and 4mL solvent toluene into a thick-walled pressure-resistant reaction bottle, and put it into a 120 o After reacting 7h in C oil bath, monitor reaction by thin-layer chromatography completely, by 1 H-NMR internal standard method was used to determine the yield of the target product, and the yield of the target product citral was 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com