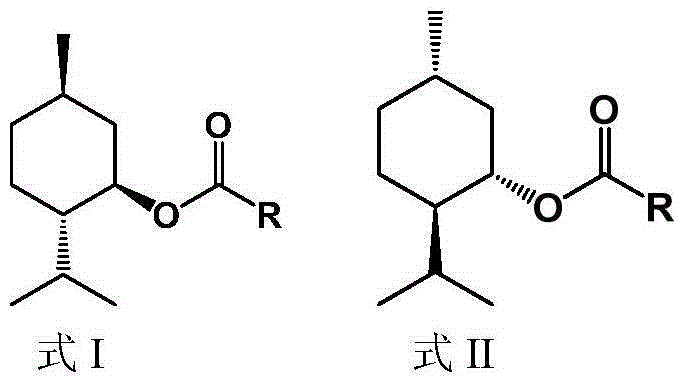
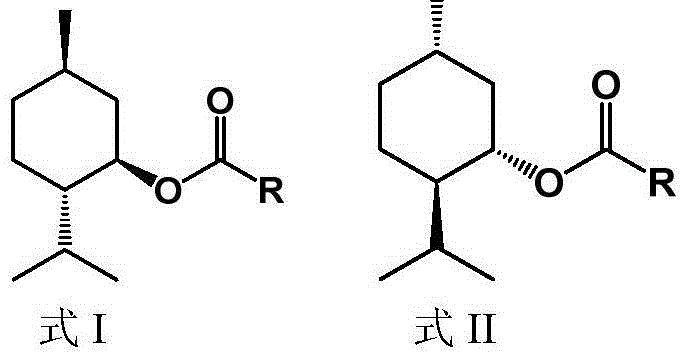
Method for splitting D,L-menthol
A technology of menthol and menthyl esters, applied in the field of splitting D, can solve the problems of difficult industrial application, limited resolution concentration, low yield of menthyl esters, etc., and achieve the effect of facilitating industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) 1 g of D-menthyl benzoate with an optical purity of 40% as a substrate was dissolved in 8 mL of ethanol at 25°C, and after stirring for 30 minutes, the temperature was lowered to 0°C and stirred for 30 minutes to precipitate 260 mg of solid.
[0028] (2) Add 100 mg of the solid to a 2mol / L sodium hydroxide aqueous solution, and heat and stir at 100°C for 6 hours to perform alkaline hydrolysis to obtain D-menthol. The hydrolysate was subjected to gas phase analysis, and the optical purity of D-menthol was 98.1%.
Embodiment 2
[0030] 1 g of D-menthyl benzoate with an optical purity of 60% as a substrate was dissolved in 9 mL of ethanol at 30°C, and after stirring for 30 minutes, the temperature was lowered to 5°C and stirred for 30 minutes to precipitate 400 mg of solid.
[0031] Take 100mg of the solid and add it to a 1mol / L sodium hydroxide aqueous solution, and heat and stir at 100°C for 8 hours for alkaline hydrolysis to obtain D-menthol. The hydrolysate was subjected to gas phase analysis, and the optical purity of D-menthol was 98.5%.
Embodiment 3
[0033] 1g of D-menthyl benzoate with an optical purity of 90% as the substrate, dissolved in 8 mL of ethanol at 35°C, stirred for 30 minutes, cooled to 0°C, stirred for 30 minutes, and added optically pure D-menthyl benzoate Seed crystals were 30 mg, and stirring was continued for 30 minutes, 460 mg of solids were precipitated.
[0034] Take 100 mg of the solid and add it to a 2mol / L potassium hydroxide aqueous solution, and heat and stir at 100°C for 5 hours for alkaline hydrolysis to obtain D-menthol. The hydrolysate was subjected to gas phase analysis, and the optical purity of D-menthol was 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com