A kind of green synthesis method of 1 naphthylamine
A technology of green synthesis and naphthylamine, which is applied in the preparation of amino groups instead of hydrogen atoms, can solve the problems of long process, environmental pollution, and high cost, and achieve the effects of short process, improved conversion efficiency, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Take by weighing 2.5g of hydroxylamine hydrochloride, drop into the three-necked flask that is connected with thermometer, condenser tube, then measure 12mL glacial acetic acid and 3mL deionized water and join in the three-necked flask, then place the three-necked flask in a constant temperature water bath, at 30 After stirring at a constant temperature for 20 minutes at °C, 0.5 g of naphthalene was added, the temperature was raised to 80 °C, and the reaction was stopped after 12 hours of constant temperature reaction. After post-processing the reaction solution, 0.54 g of the final product was obtained. The mass of 1-naphthylamine in the product was quantitatively analyzed by liquid chromatography external standard method to be 14.5 mg, and the yield was 2.64%.
Embodiment 2
[0031] Take by weighing 2.5g of hydroxylamine hydrochloride, drop into the three-necked flask that is connected with thermometer, condenser tube, then measure 12mL glacial acetic acid and 3mL deionized water and join in the three-necked flask, then place the three-necked flask in a constant temperature water bath, at 30 After stirring at a constant temperature for 20 minutes at ℃, 0.5 g of naphthalene was added, the temperature was raised to 80 ℃, and the reaction was stopped after 24 hours of constant temperature reaction. After post-processing the reaction solution, 0.54 g of the final product was obtained. The quality of 1-naphthylamine in the product was quantitatively analyzed by liquid chromatography external standard method, and the yield was 8.09%.
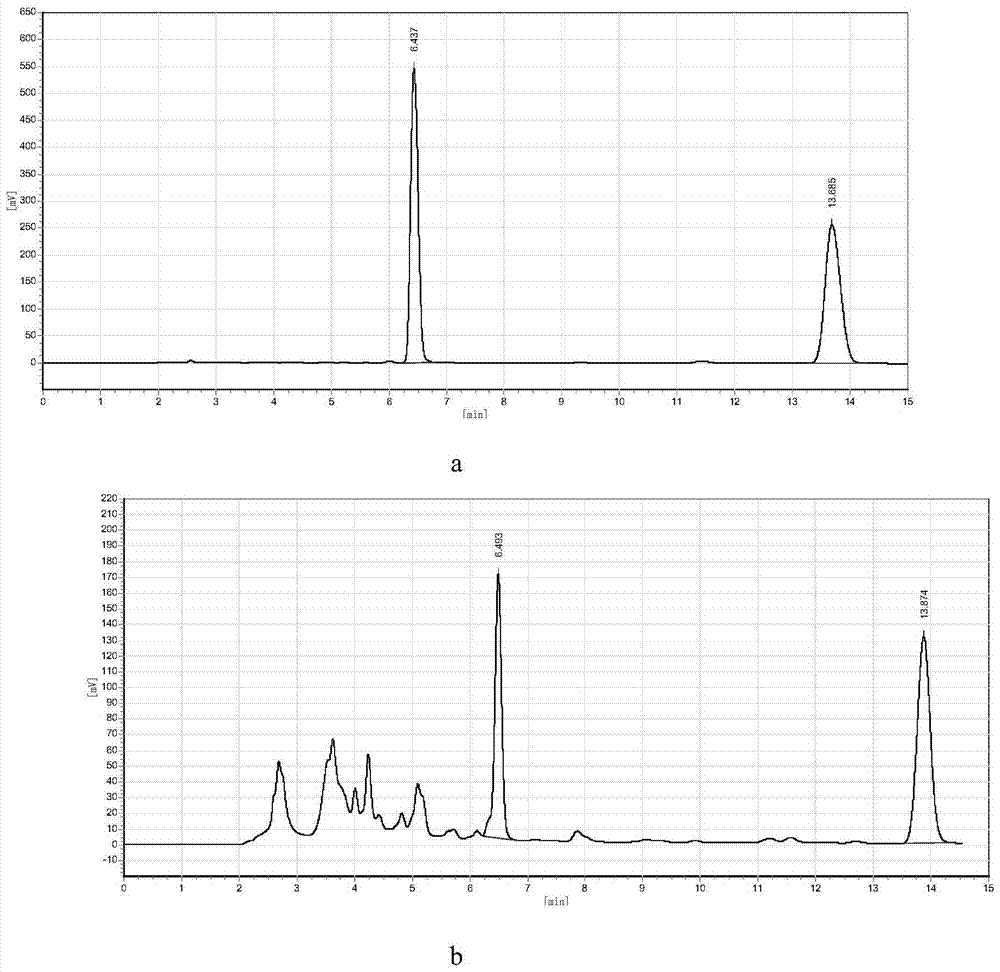
[0032] Analysis from liquid chromatography figure 1 As can be seen in (b), the reaction generates 1-naphthylamine, wherein the retention time of 1-naphthylamine is about 6.4 minutes, and the retention time of raw material...
Embodiment 3
[0038] Weigh 0.025g of sodium metavanadate and 1.0g of hydroxylamine hydrochloride respectively, put them into a three-necked flask connected with a thermometer and a condenser tube, then measure 15mL of acetic acid and 5mL of deionized water into the three-necked flask, and then place the three-necked flask on In a constant temperature water bath, stir at 30°C for 20 minutes, then add 0.5 g of naphthalene, raise the temperature to 80°C, and stop the reaction after 2 hours of constant temperature reaction. After post-processing the reaction solution, 0.58 g of the final product was obtained. The mass of 1-naphthylamine in the product was quantitatively analyzed by liquid chromatography external standard method to be 61.5 mg, and the yield was 11%.
[0039] From Example 1, Example 2 and Example 3, it can be seen that the addition of the catalyst can obviously promote the reaction and improve the productive rate of 1-naphthylamine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com